Abstract
Oocyte maturation arrest results in primary female infertility, but the genetic etiology of this phenotype remains largely unknown. Previously, we and other groups have reported that biallelic mutations in PATL2 are mainly responsible for human oocyte germinal vesicle-stage arrest and that the specific phenotype varies for different mutations. Here, we identified four novel missense mutations (p.V260M, p.Q300*, p.T425P, and p.D293Y), a novel frameshift mutation (p.N239Tfs*9), and a reported splicing mutation (p.R75Vfs*21) in PATL2 in seven affected individuals from five unrelated families, showing a multiplicity of phenotypes in oocyte maturation arrest, fertilization failure, or embryonic developmental arrest, which further expands the mutational and phenotypic spectrum in patients with PALTL2 mutations. This work further indicates the critical role of PATL2 in oocyte maturation and early embryo development and will provide a basis for pursuing the determination of genetic variation in PALT2 as an additional criterion for evaluating the quality of oocytes and embryos for assisted reproduction techniques.
Similar content being viewed by others
Introduction
Infertility is a relatively common disorder of the reproductive system and affects 10.7–15.15% of couples [1]. The occurrence of infertility remains unexplained in many cases, and a molecular understanding of infertility has the potential to reveal fundamental insights into human reproduction. Successful human reproduction starts from the fusion of a sperm with a mature oocyte, and there is a series of distinct morphological and molecular events that occur during oocyte maturation, including germinal vesicle (GV) breakdown, meiotic spindle assembly, polarized differentiation of the oocyte cortex, and asymmetric division to extrude the first polar body [2,3,4]. Abnormalities in any of these events will result in oocyte maturation arrest and subsequent fertilization failure or early embryonic developmental arrest, which ultimately will lead to primary female infertility.
Human oocyte maturation arrest was first described in 1990 [5], and three types of oocyte anomalies were observed during in vitro fertilization (IVF): GV arrest, metaphase I (MI) arrest, and the absence of oocytes. Similar cases were subsequently reported [6,7,8,9], but the genetic factors related to oocyte maturation arrest were rarely studied and remained largely unknown. Feng et al. first determined the inheritance pattern of human oocyte MI arrest and identified TUBB8 mutations, which are responsible for the disease [10,11,12]. Recently, four studies have demonstrated that biallelic mutations in PATL2 are primarily responsible for human oocyte GV arrest. The first study initially identified a homozygous nonsense mutation of PATL2 (c.784C>T [p. Arg262*]) in a consanguineous family affected by human oocyte GV arrest. The subsequent mutation screening of PATL2 in a cohort of 179 individuals identified four unrelated subjects with compound heterozygous PATL2 mutations with a slight phenotypic variability [13]. In the second study, two homozygous PATL2 variants were identified in two oocyte GV arrest subjects from two different Saudi Arabian families [14]. In the latest two studies, biallelic mutations in PATL2 were identified in two independent cohorts accounting for about 26% (6/23) and 44% (4/9) of typical oocyte GV arrest patients, respectively [15, 16].
Here, we screened PATL2 mutations in patients with primary infertility who had been previously diagnosed with oocyte maturation arrest, low fertilization rates, or early embryonic arrest. Seven new patients from five unrelated families were detected harboring PATL2 mutations, including four novel missense mutations (p.V260M, p.Q300*, p.T425P, and p.D293Y), a novel frameshift mutation (p.N239Tfs*9), and a reported splicing mutation (p.R75Vfs*21) causing a variety of clinical phenotypes. This finding further indicates the critical role of PATL2 in oocyte maturation and early embryo development.
Materials and methods
Human subjects
A total of 216 affected individuals with oocyte maturation arrest, low fertilization, or embryonic development arrest were screened and seven patients from five unrelated families were identified in possible pathogenic mutations. DNA samples from the affected patients and their family members were obtained, and all of the affected individuals had a normal chromosomal karyotype. The seven primary infertility patients were diagnosed with oocyte maturation arrest, low fertilization, or embryonic development arrest.
Mutational screening of PATL2
Genomic DNA samples of affected individuals, their family members, and controls were extracted from peripheral blood using standard methods (QIAamp DNA Blood Mini Kit; Qiagen, Hilden, Germany). All exons and splicing sites of PATL2 were amplified, and the corresponding primers are listed in Table 1. Amplified fragments were purified using ExoSAP-IT™ PCR Product Cleanup Reagent (78201.1.ML; Applied Biosystems) and directly sequenced using an ABI 3100 DNA analyzer (Applied Biosystems, Foster City, CA, USA). PCR conditions were as follows: pre-denaturing at 94 °C for 5 min, followed by 35 cycles of denaturing at 94 °C for 30 s, annealing at 60 °C for 30 s, and extending at 72 °C for 1 min, and a final elongation at 72 °C for 5 min.
Analysis of sequence variants in PATL2
The PATL2 sequence was aligned using the CodonCode software (CodonCode Co., USA) to identify rare variants. The Exome Aggregation Consortium browser (ExAC database, http://exac.broadinstitute.org/) was used to determine the frequency of the corresponding mutations. Analysis of mutations was performed by using Sorting Intolerant From Tolerant (SIFT, http://sift.bii.a-star.edu.sg/), Mutation Taster (http://www.mutaiontaster.org/), and Polymorphism Phenotyping (Polyphen-2, http://genetics.bwh.harvard.edu/pph2/).
Evaluation of oocyte and embryo phenotypes
Oocytes were obtained from the affected individuals and controls undergoing clinical intracytoplasmic sperm injection (ICSI). The morphologies of oocytes, fertilization, and embryonic development were evaluated by light microscopy with an Olympus IX71 inverted microscope system. Oocyte immunostaining was performed as previously described [16]. Briefly, oocytes were fixed in 2% paraformaldehyde containing 0.1% bovine serum albumin. Oocytes were then incubated in membrane-permeabilizing solution (0.5% triton in phosphate-buffered saline) for 20 min and blocking buffer (0.1% Tween 20, 0.01% triton, and 1% bovine serum albumin in phosphate-buffered saline) for 2 h. The oocyte PATL2 was stained with an anti-PATL2 antibody (1:300 dilution, ab170827; Abcam), and Hoechst 33342 (1:600 dilution; BD Biosciences, USA) was used to label the DNA. For the immunostaining of the negative control, the oocyte was treated as above but incubation with the primary antibody was omitted. Finally, whole-mount oocytes on glass slides were examined by confocal microscopy (Leica TCS SP8; Germany) with an excitation wavelength of 405 nm.
Results
Clinical characteristics and phenotypes of patients
All patients had had primary infertility over the past few years, and their spouses had normal sperm counts with normal morphology and motility. Most of the affected individuals had experienced several failed IVF/ICSI attempts.
Both of the two patients (II-1 and II-2) from family 1 were sisters and had suffered from primary infertility for 4 and 9 years, respectively. For patient II-1, 11 oocytes were retrieved, and all were arrested at the GV stage. For patient II-2, nine oocytes were retrieved and six were arrested at the GV stage.
In family 2, patients II-1 and II-2 were also sisters and had been diagnosed with primary infertility for 4 and 6 years, respectively. Patient II-1 experienced two IVF/ICSI cycles, and 24 oocytes were retrieved, of which 6 were arrested at the GV stage and the remaining were abnormal, including morphological abnormalities and degeneration (Fig. 2a). Patient II-2 had a similar phenotype as patient II-1, with 6 of 15 oocytes arrested at the GV stage and the remaining 9 oocytes having abnormal morphology (Fig. 2a).
Patients in families 3–5 had similar clinical phenotypes—most of the retrieved oocytes exhibited low fertilization rates and embryonic developmental arrest. Patient II-1 from family 3 experienced four IVF/ICSI cycles, and in the first three attempts (with unknown oocyte stages because information from the hospital was unavailable), only 2 out of 36 oocytes cleaved to form embryos, and the pregnancy failed. In the fourth ICSI cycle, six metaphase II (MII) oocytes were retrieved, and only one could be fertilized and cleaved. However, there is no pregnancy after embryo transfer (Table 3). Patient II-1 in family 4 underwent IVF in her first cycle, but fertilization failed for the five retrieved MII oocytes as indicated by the absence of pronuclei. In the second and third ICSI attempts, the patient had a high proportion of MII oocytes (16 MII oocytes out of 23 oocytes). Of these MII oocytes, only one could be successfully fertilized, while all the others did not exhibit pronuclei. The fertilized oocyte resulted in a cleaved embryo, but the pregnancy failed after embryo transfer (Table 3). Patient II-1 in family 5 also had a high proportion of MII oocytes (17 MII oocytes out of 19 oocytes), and 10 zygotes were obtained, but all embryos were arrested at an early stage. The clinical characteristics of the oocytes retrieved from the patients are summarized in Table 3.
Identification of novel mutations in PATL2
Altogether, we identified four novel missense mutations, including a novel frameshift mutation, and a reported splicing mutation in PATL2. Patients from families 1 and 5 carried biallelic mutations c.778G>A (p.V260M) and c.223–14_223–2del (p.R75Vfs*21). Patients II-1 and II-2 from family 1 were sisters, and the biallelic mutations p.V260M and p.R75Vfs*21 in these two patients were inherited from their father and mother, respectively (Fig. 1a). The patient in family 5 had an unknown inheritance pattern because information about her parents was unavailable (Fig. 1a). The two patients in family 2 were also sisters and carried the compound heterozygous missense mutations c.898C>T (p.Q300*) and c.1273A>C (p.T425P), and the p.Q300* and p.T425P mutations were inherited from their mother and father, respectively. The patient in family 3 carried compound heterozygous mutations c.887G>T (p.D293Y) and c.223–14_223–2del (p.R75Vfs*21) with an unknown inheritance pattern. The patient in family 4 carried compound heterozygous deletion mutations c.716delA (p.N239Tfs*9) and c.223–14_223–2del (p.R75Vfs*21), which were inherited from her mother and father, respectively (Fig. 1a). The four missense mutations (p.V260M, p.D293Y, p.Q300*, and p.T425P) and one frameshift mutation (p.N239Tfs*9) are novel mutations that have not been reported previously. Specific information on the location of the mutations, their frequency, and their in silico analysis is provided in Table 2. Clinical information for the patients is given in Table 3. The locations of the mutations and conservation among different species are indicated in Fig. 1b.
Identification of mutations in PATL2. a Pedigrees of five families with mutations in PATL2. Four novel missense mutations, a novel frameshift mutation, and a reported splicing mutation in PATL2 were identified in five unrelated families. The patients in families 1 and 5 had the same compound heterozygous V260M and R75Vfs*21 mutations. Compound heterozygous mutations D293Y and R75Vfs*21 were identified in family 3. Both sisters from family 2 had compound heterozygous missense mutations Q300X and T425P. The patient in family 4 had compound heterozygous N239Tfs*9 and R75Vfs*21 deletion mutations in PATL2. The mutations in the affected individuals from families 1, 2, and 4 were inherited from their parents. The patients in families 3 and 5 had unknown inheritance patterns. b Location and conservation of mutations in PATL2.The positions of all mutations are indicated in the genomic structure of PATL2. Two mutations are located in exon 8, two mutations are located in exon 10, and one mutation is located in exon 13. The affected amino acids were compared among seven mammalian species in a conservation analysis. The “ = ” sign indicates infertility, and the black circles represent the affected individuals
Expression of PALT2 in the patient’s oocyte from family 2
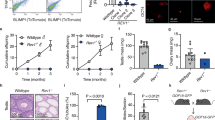
To evaluate the effect of p.Q300* and p.T425P mutations on the amount of PATL2 that is expressed, we used fixed GV oocytes for immunostaining from patient II-1 in family 2, who had a small number of GV oocytes and who had a high proportion of abnormal oocytes. PATL2 was mostly located in the cytoplasm of both normal GV oocytes and the affected individuals’ oocytes, but the affected individuals’ GV oocytes showed extremely reduced amounts of PATL2, implying that the amount of PATL2 was greatly impaired by mutations p.Q300* and p.T425P, which is consistent with the previous findings [13] (Fig. 2b).
Phenotype of oocytes retrieved from the patients. a The morphology of normal and affected individuals’ germinal vesicle (GV), metaphase I (MI), metaphase II (MII), and abnormal oocytes. Patients from family 2 had a high proportion of abnormal oocytes and a small number of GV oocytes. b Immunolabeling of normal and affected family 2 individuals’ GV oocytes. Oocytes were immunolabeled with antibodies against PATL2 (shown in green) for visualization of the protein distribution and were counterstained with Hoechst 33342 (shown in blue) for DNA visualization. The morphologies of oocytes were examined with an inverted microscope system (OLYPUS IX71), and the immunolabeling was examined by confocal microscopy (Leica). PATL2 was mostly located in the cytoplasm of the normal GV oocyte and the affected individuals’ GV oocyte, but the affected individuals’ GV oocyte showed reduced fluorescence compared to the normal oocyte, implying that the amount of PATL2 was greatly impaired by p.Q300* and p.T425P mutations. For negative control (NC), the oocyte was stained without primary antibody. The scale bar represents 50 µM
Discussion
In this study, we identified four novel missense mutations, a novel frameshift mutation, and a reported splicing mutation in PATL2, in seven affected individuals from five unrelated families, and these expand upon the known genetic variants for the disease. Consistent with the previous findings [13], patients with different PATL2 mutations showed a multiplicity of phenotypes in terms of oocyte maturation arrest, fertilization failure, or embryonic developmental arrest.
The five independent families with different mutations showed clearly different clinical phenotypes. The majority of the oocytes retrieved from the two patients in family 1 were arrested at the GV stage, which was consistent with the typical phenotype resulting from the previously reported PATL2 mutation [14,15,16]. It is worth noting that the affected individuals from families 1 and 5 carried the same compound heterozygous mutations (V260M and R75Vfs*21) but showed different phenotypes, with typical GV-stage arrest in family 1 and early embryonic developmental arrest in family 5. This might be the result of cis-regulatory variation, which in turn results in modified penetrance [17]. These findings showed that different mutations in PALT2 can result in variability in the phenotypes of oocytes/embryos, including oocytes arrested at the GV stage and degenerated oocytes, low fertilization rates, or early embryonic arrest. The clinical phenotypic variability in the patients who carried compound heterozygous mutations in PATL2 most likely resulted from the fact that different mutations have different types of effects, which is consistent with previous finding [13].
During oocyte growth, a large amount of stable mRNA necessary for growth and maturation accumulates in the oocytes. Most of the mRNA that is synthesized is immediately translated to support the growing oocyte, but up to 30% of the mRNAs are stored for subsequent translation, meiotic resumption, or early zygote development [18]. A number of RNA-binding proteins control mRNA stability [19], and PATL2 was initially treated as an mRNA-binding protein associated with other mRNA-binding proteins such as CPEB, Xp54, and xRAP55 [20, 21]. It has been suggested that PATL2 might be involved in a novel pathway controlling the stability of specific mRNAs in mice and that it might be a vital player in oocyte growth and maturation by regulating the expression of mRNAs encoding proteins that are crucial for oocyte meiotic progression and early embryonic development [15]. In the present study, compared to the control oocytes, the affected individuals’ oocytes showed extremely reduced amounts of PATL2 (Fig. 2b). Mutations in PATL2 might reduce PATL2 expression to different extents and thereby result in different clinical phenotypes. The function of PATL2 remains largely unknown, and the exact function of PATL2 in human oocytes should be investigated in the future.
Recently, four reports have been published describing mutations in PATL2 that are involved in human oocyte GV-stage arrest [13,14,15,16]. Here, we identified an additional five novel PATL2 mutations and a reported splicing mutation in five unrelated families showing different clinical phenotypes, and this further expands the mutational and phenotypic spectrum in patients with PATL2 mutations. This work will provide a basis for pursuing the determination of genetic variation in PATL2 as an additional criterion for evaluating the quality of oocytes and embryos. Uncovering the genetic and molecular basis of oocyte maturation arrest will help patients by improving diagnosis and our understanding of their disease, which will facilitate the success of IVF/ICSI procedures.
References
Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99:1324–31.
Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4.
Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–9.
Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update. 2015;21:427–54.
Rudak E, Dor J, Kimchi M, Goldman B, Levran D, Mashiach S. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril. 1990;54:292–6.
Hartshorne G, Montgomery S, Klentzeris L. A case of failed oocyte maturation in vivo and in vitro. Fertil Steril. 1999;71:567–70.
Levran D, Farhi J, Nahum H, Glezerman M, Weissman A. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod. 2002;17:1604–9.
Beall S, Brenner C, Segars J. Oocyte maturation failure: a syndrome of bad eggs. Fertil Steril. 2010;94:2507–13.
Hourvitz A, Maman E, Brengauz M, Machtinger R, Dor J. In vitro maturation for patients with repeated in vitro fertilization failure due to “oocyte maturation abnormalities”. Fertil Steril. 2010;94:496–501.
Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med. 2016;374:223–32.
Feng R, Yan Z, Li B, Yu M, Sang Q, Tian G, et al. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet. 2016;53:662–71.
Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, et al. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod. 2017;32:457–64.
Chen B, Zhang Z, Sun X, Kuang Y, Mao X, Wang X, et al. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet. 2017;101:609–15.
Maddirevula S, Coskun S, Alhassan S, Elnour A, Alsaif HS, Ibrahim N, et al. Female infertility caused by mutations in the oocyte-specific translational repressor PATL2. Am J Hum Genet. 2017;101:603–8.
Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blévec E, Karaouzène T, Conne B, et al. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med. 2018;10:e8515.
Huang L, Tong X, Wang F, Luo L, Jin R, Fu Y, et al. Novel mutations in PATL2 cause female infertility with oocyte germinal vesicle arrest. Hum Reprod. 2018;33:1183–90.
Castel SE, Cervera A, Mohammadi P, Aguet F, Reverter F, Wolman A, et al. Modified penetrance of coding variants by cis-regulatory variation contributes to disease risk. Nat Genet. 2018;50:1327–34.
Piqué M, López JM, Foissac S, Guigó R, Méndez R. A combinatorial code for CPE-mediated translational control. Cell . 2008;132:434–48.
Clarke HJ. Post-transcriptional control of gene expression during mouse oogenesis. Results Probl Cell Differ. 2012;55:1–21.
Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–29.
Nakamura Y, Tanaka KJ, Miyauchi M, Huang L, Tsujimoto M, Matsumoto K. Translational repression by the oocyte-specific protein P100 in Xenopus. Dev Biol. 2010;344:272–83.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (81771574, 81771649, 81300550, 81725006, 81822019, 81771581, and 81571501), the National Key Research and Development Program of China (2017YFC1001500, 2016YFC1000600, and 2018YFC1003800), the National Basic Research Program of China (2015CB943300), the Shanghai Municipal Commission of Health and Family Planning Foundation (20174Y0036), and the Shanghai Rising-Star Program 17QA1400200.
Author contributions
LW, BL and QS: literature review, collection of data, and drafting the manuscript; HC, DL, DS, BBC and ZY: data collection; QFL, LW, YPK, BL and QS: supervision of all aspects, including study design, data interpretation, and manuscript preparation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee of Ninth Hospital affiliated with Shanghai Jiao Tong University (No. 20161206; approved on 5 December 2016). All immature oocytes were donated by affected individuals after they had provided written informed consent, and the control MII oocytes used in this study were matured in vitro from GV or MI oocytes.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, L., Chen, H., Li, D. et al. Novel mutations in PATL2: expanding the mutational spectrum and corresponding phenotypic variability associated with female infertility. J Hum Genet 64, 379–385 (2019). https://doi.org/10.1038/s10038-019-0568-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0568-6
This article is cited by
-
Identification novel mutations and phenotypic spectrum expanding in PATL2 in infertile women with IVF/ICSI failure
Journal of Assisted Reproduction and Genetics (2024)
-
Homozygous variants in CDC23 cause female infertility characterized by oocyte maturation defects
Human Genetics (2023)
-
Advances in the study of genetic factors and clinical interventions for fertilization failure
Journal of Assisted Reproduction and Genetics (2023)
-
Molecular tools for the genomic assessment of oocyte’s reproductive competence
Journal of Assisted Reproduction and Genetics (2022)
-
Novel mutations in NLRP5 and PATL2 cause female infertility characterized by primarily oocyte maturation abnormality and consequent early embryonic arrest
Journal of Assisted Reproduction and Genetics (2022)