Abstract
Multichannel near-infrared spectroscopy (MNIRS) was used for the functional imaging of the sensorimotor cortex of newborn infants during passive knee and elbow movement under sedated sleep. Contralateral knee and elbow movement caused a marked increase in the concentration of oxyhemoglobin ([oxyHb]) from the baseline values at site within the sensorimotor area in all infants. During ipsilateral knee and elbow movement, [oxyHb] showed smaller changes, equivalent to 64 ± 23 and 66 ± 28% of the changes that occurred with contralateral stimulation, respectively. The mean times corresponding to maximal changes in [oxyHb] were 16.1 ± 3.3 s for contralateral knee movement and 17.9 ± 5.7 s for contralateral elbow movement. No significant difference was noted between the mean latencies showing the maximal changes in [oxyHb] between contralateral and ipsilateral movement. There was a significant difference in the area and degree of response between the contralateral and ipsilateral movement. MNIRS could be a useful tool to understand the pathophysiology of the developing brain and monitor cortical responses in various clinical situations.
Similar content being viewed by others
Main
During development, the brain undergoes sequential anatomical, functional, and organizational changes necessary to support the complex adaptive behavior of a mature normal individual. The delineation of developmental changes occurring in different brain regions would provide a means of relating various behavioral phenomena to maturation-specific brain structures. Advancing our understanding of cerebral structure-function relationships in the neonatal period is useful for the early prediction of concomitant motor sequelae in infants. We hypothesized that reliable and early cerebral functional assessments are useful for tailored early intervention and rehabilitation programs in NICUs or as a part of early home intervention to maximize recovery in infants with brain damage, especially regarding assessment of the effectiveness of intervention and rehabilitation programs. Therefore, we think that it is important to investigate the process of specialization of the brain to form functional areas in one hemisphere (lateralization) to understand reorganization after brain injury.
Physiologic studies of lateralization have been reported involving the frontal and temporal cortex of premature infants (1–3), term infants (4,5), and 4-mo-old infants (6,7). These studies used EEG, evoked potential, functional magnetic resonance imaging (fMRI), and near-infrared optical imaging to detect the differences in cortical activation between the left and right regions in the frontal and temporal cortex.
Near-infrared spectroscopy (NIRS) is a noninvasive method for detecting changes in the concentrations of oxyhemoglobin ([oxyHb]), deoxyhemoglobin ([deoxyHb]), and total hemoglobin ([totalHb]). NIRS has been used to study functional activations of various regions of the cortical area in the brain. This is based on the assumption that an increase in recorded [oxyHb] represents an increase in blood flow, which, in turn, reflects neural activation. Most studies in which NIRS was used to assess cortical function have involved adult subjects. However, NIRS is more easily applicable for neonates because the light more readily permeates the neonatal head compared with the adult head because of its small size, and the measured values are less influenced by layered structures, such as the scalp, skull, and cerebrospinal fluid. In newborn infants, NIRS has been used to assess the activities of the visual cortex (8–10), frontal cortex (11), temporal cortex (7,12), and olfactory cortex (13) after repeated light simulation, music stimulation, verbal stimulation, and odor stimulation, respectively. Especially, NIRS has been used to mainly asses the activation of the visual cortex induced by checkerboard or flashlight stimulation in awake (8,14) and sleeping (9,10,15–17) and at different ages ranging from 32 wk of gestation to 4 mo. However, only a few studies have been conducted on the somatosensory cortex in infants under passive motor stimulation (1,2).
In this study, we used multichannel NIRS (MNIRS) to monitor the activities of the sensorimotor cortex as mirrored by hemodynamic responses in newborn infants subjected to passive unilateral knee and elbow joint movement during sedated sleep, and we investigated the differences between cortical responses with such passive knee and elbow movements. We previously demonstrated that this MNIRS method is feasible and safe in sensorimotor cortex studies involving sedated preterm infants (1).
METHODS
The study was conducted involving two term infants and eight preterm infants (GA, 24–41 wk; median age, 29.6 wk) on d 3–99 after birth (median, d 6.0). Written informed consent was obtained from the parents of the infants, and the protocol was approved by the ethical committee, Faculty of Medicine, Kagawa University. Clinical data on the infants are shown in Table 1.
We used a 24 multichannel NIRS with 8 light-incident fibers and 8 detector fibers, each with an interoptode distance of 2 cm (Hitachi Medico Co., Japan). The light sources were two 0.5-mW continuous laser diodes with wavelengths of 780 and 830 nm, respectively. The probes were placed in the left or right parietotemporal region over the sensorimotor cortex.
Functional imaging tests were performed on infants who had been sedated by the i.v. injection of thiamylal sodium (5 mg/kg) to prevent spontaneous movement. An examiner then flexed and extended the infant's knee joint or elbow joint at a frequency of ∼0.7 Hz. All infants were monitored by pulse oximetry during the examination. The infants were clinically in quiet sleep, although the sleep state was not systematically assessed by EEG. This passive motor stimulation consisted of 10 to 12 repetitions of movement, each lasting 15 s, followed by a 30-s rest period. Prebaseline data were collected for 5 s. Data were collected every 0.1 s, averaged over 8 cycles, and smoothed. We measured the changes in [oxyHb], [deoxyHb], and [totalHb] from prebaseline values.
Means and standard deviations of changes in each parameter were calculated from 8 to 10 trials in which continuous stable measurements were possible. Changes in variables were determined from the means of values measured during a 5-s period from the start of measurement, with the start and endpoints of measurement (2.5 s before onset of the stimulus and 32.5 s after end of the stimulus, respectively) set to zero. From the 24 source-detector signals, the signal with the greatest change in [oxyHb] in each subject was selected for group statistical analysis. Wilcoxon signed-rank test was used for statistical analysis of changes in parameters from knee and elbow stimulation, and p values < 0.05 were considered significant. For the analysis of activated areas in each subject, the Mann-Whitney U test was used for the statistical analysis of differences in [oxyHb] for all tasks in the prestimulation periods (5 s) and during or after stimulation periods (5 s with the greatest change in [oxyHb] from 24 channels).
RESULTS
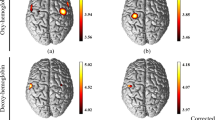
Figure 1 shows topographical image patterns of grand averages of [oxyHb], [deoxyHb], and [totalHb] during knee or elbow stimulation in an infant. These figures show changes in focal brain hemodynamics in the sensorimotor cortex. Contralateral knee movement caused a marked increase in [oxyHb] and [totalHb] from the baseline values at almost all locations in the sensorimotor area and a decrease in [deoxyHb]. During ipsilateral knee movement, [oxyHb] and [totalHb] showed smaller changes at a few locations compared with contralateral knee movement. Contralateral elbow movement caused a marked increased in [oxyHb] and [totalHb] in a more occipital area than knee movement. However, the other subjects did not show the same activation pattern. During ipsilateral elbow movement, [oxyHb] and [totalHb] showed smaller changes at a few locations compared with contralateral elbow movement. Figure 2 shows the grand average of concentration changes for an infant as a function of time. [oxyHb] increased gradually with contralateral knee and elbow movement until reaching a peak at 15 and 18 s, respectively, and then slowly returned to the baseline level after the passive stimulus stopped. The changes in [totalHb] were nearly the same as those in [oxyHb]. The decrease in [deoxyHb], in contrast, began later than the changes in [oxyHb] and continued even after the stimulation stopped. During ipsilateral knee and elow movement, [oxyHb] showed smaller changes at a few locations, equivalent to 42 and 37% of the changes that occurred with contralateral movement, respectively.
The grand average of concentration changes in one subject as a function of time. The average from 10 trials in an infant (case 5). (A) The results of channel 6 during contralateral knee stimulation, (B) the results of channel 2 during ipsilateral knee stimulation, (C) the results of channel 5 during contralateral elbow stimulation, and (D) the results of channel 9 during ipsilateral elbow stimulation. The arrow indicates the 15-s passive sensorimotor stimulation period. Changes in [oxyHb], [deoxyHb], and [totalHb] are given in mM·mm. The red, blue, and green line indicates [oxyHb], [deoxyHb], and [totalHb], respectively.
Table 2 shows changes in [oxyHb], [deoxyHb], and [totalHb] in response to knee or elbow sensorimotor stimulation in all infants. In all infants, there were increases in [oxyHb] and [totalHb]. The average changes in [oxyHb] during the respective peak responses were 0.078 ± 0.051 (SD) mM·mm for contralateral knee movement, 0.047 ± 0.022 mM·mm for ipsilateral knee movement, 0.084 ± 0.0 64 mM·mm for contralateral elbow movement, and 0.058 ± 0.023 mM·mm for ipsilateral elbow movement. During ipsilateral knee and elbow movement, [oxyHb] showed weaker changes, equivalent to 64 ± 23 and 66 ± 28% of the changes that occurred with contralateral movement, respectively. The average changes in [oxyHb] during ipsilateral knee or elbow movement were significantly lower than during contralateral knee or elbow movement. The mean times corresponding to maximal changes in [oxyHb] were 11.1 ± 3.3 s for contralateral knee, 12.2 ± 3.2 s for ipsilateral knee, 12.9 ± 5.7 s for contralateral elbow, and 9.1 ± 3.3 s for ipsilateral elbow movement, respectively. The mean time corresponding to maximal changes in [oxyHb] were not significant between contralateral and ipsilateral knee elbow movement.
Figure 3 shows a summary of individual p values for [oxyHb] between the prestimulation and stimulation periods for all tasks in all 24 channels, and directions of changes in [oxyHb], either increases or decreases. Knee joint movement caused a marked increase in [oxyHb] in a larger or the same area of the contralateral than ipsilateral area in cases 1, 3, 4, 5, 7, and 10. Elbow joint movement caused a marked increased in [oxyHb] in a larger or the same area of the contralateral than ipsilateral area in cases 2, 5, 6, 7, and 10.
Summary of individual p values for [oxyHb] between the prestimulation and stimulation periods of all tasks in all channels. RT, right hemisphere measurement; LT, left hemisphere measurement. Number indicates measurement channel number. Red and blue channels in each panel indicate increments and decrements of [oxyHb], respectively. The intensity of the color shows p values; the thin line, bold line, and solid color indicates p < 0.05, p < 0.01, and p < 0.001, respectively.
DISCUSSION
This study revealed that the bilateral sensorimotor area function in response to passive motor stimulation of the knee and elbow joint can be imaged and evaluated even in newborn infants during sleep under sedation. The results showed that the typical responses in sensorimotor area cortex activation are an increase in [oxyHb] and [totalHb] and a slight decrease in [deoxyHb]. Villringer and Chance (18) described the significance of [oxyHb] and [deoxyHb] changes during neural activation. They reported that there is a decrease in [oxyHb] and corresponding increase in [deoxyHb] for a few seconds after stimulus onset, in which oxygen is consumed. However, such changes were not observed in this study. Next, when regional cerebral blood flow increased, there was an increase in [oxyHb] and often a decrease in [deoxyHb] because of washout. The relative contribution of these two effects determines whether [deoxyHb] increases or decreases, as was confirmed by the results of this study.
In this study, the mean times corresponding to maximal changes in [oxyHb] were 11.1 s for contralateral knee, 12.2 s for ipsilateral knee, 12.9 s for contralateral elbow, and 9.1 s for ipsilateral elbow movement, respectively. No significant difference was noted between the mean latencies showing the maximal changes in [oxyHb] between the contralateral and ispilateral movements. Studies of the response in the sensorimotor cortex to a motor task in adult humans using NIRS showed that [oxyHb] reached a peak at about 6–8 s after the start of the task (19,20). Compared with results using adult subjects, these response times are slower. The difference in the response times of infants and adults reflects a different functional organization of the sensorimotor cortex in infants or on-going myelin and synapse development and neurovascular coupling. In an animal study of the somatosensory cortex in rats, by comparing fMRI measurements with electrophysiological recordings, advancing age was associated with an increase in the amplitude of the blood oxygenation level-dependent (BOLD) signal and a decrease in the time to the peak of the BOLD signal from postnatal d 13 to adulthood (21). The maturation of the hemodynamic response was correlated with the age-dependent increases in susceptibility to inhibition of carbonic anhydrase. These results suggest that dynamic neurovascular coupling is genuinely slower in younger animals than adults, and that both the onset and duration of the hemodynamic response are affected by age. In humans, an imaging study using positron emission tomography reported that absolute values of local cerebral metabolic rates for glucose (CMRGlc) for the sensorimotor cortex were low at birth and rapidly increased to reach adult levels by 2 y (22,23). Kinnala et al. (24) showed that CMRGlc was correlated with the postconceptional age in infants. Other studies of regional cerebral blood flow in infants also demonstrated a significant increase during this period (25–27). Furthermore, corticospinal axon conduction velocities are lower in preterm and term neonates than in older children and adult subjects (28). Thus, the cerebral maturation process may play a critical role in the hemodynamic response during passive somatosensory stimulation.
We observed ipsilateral activation of the sensorimotor cortex in infants during passive knee or elbow stimulation. Heep et al. (3) also showed that passive forearm extension and flexion in sedated preterm infants at a term-equivalent age resulted in bilateral positive or negative BOLD signal activation in the primary sensorimotor cortex, measured using fMRI. Other fMRI studies showed activation of the ipsilateral sensorimotor cortex in preterm and term infants with a mean postconceptional age of 42 wk (5,29).
In this study, there was no lateralization regarding the maximum response times of [oxyHb], but there was lateralization for areas and the degree of response during passive knee or elbow stimulation. An fMRI study (5,29) reported that passive sensory motor stimulation of the hand involving 22 neonates resulted in a slight hemispheric dominance of the somatosensory area for the contralateral side. Furthermore, a comparison of positive and negative BOLD distributions showed a stronger contralateral contribution from positive responses in both hands. These results are comparable with ours in that there is lateralization regarding the degree of response during passive stimulation. However, a comparison of the area distribution of left and right hemisphere BOLD changes with hand stimulation revealed nonsignificant hemispheric specialization (5). The reasons for the difference in the distribution of results between MNIRS and fMRI may depend on the differences of method. 1) The time resolution of MNIRS is superior to that of fMRI, but the spatial resolution of MNIRS is inferior to that of fMRI, and MNIRS also gives no information on the depth of the brain from the scalp. 2) The BOLD signal is derived from changes in [deoxyHb], but MNIRS can measure changes in [oxyHb] and [deoxyHb] separately. Then, 12 examinations in this study showed significant increase of [oxyHb] in the contralateral cortex, and eight of nine examinations showed significant increase of [oxyHb] in the ipsilateral cortex during knee stimulation. During elbow stimulation, all 12 examinations revealed a significant increase of [oxyHb] in the contralateral cortex, and four of six examinations showed significant increase of [oxyHb] in the ipsilateral cortex. These positive rates of results are higher than in an fMRI study (5). However, it is difficult to use MNIRS to detect the response in restricted cerebral regions.
The use of MNIRS is expected to facilitate investigation of the physiology of the developing brain and the brain's response to damage in premature infants, because MNIRS is a noninvasive imaging tool that allows the identification of task-related activation changes not only in the term neonatal brain but also premature brain in the NICU. However, further studies using MNIRS are needed for the neurological assessment of infants with brain damage.
This study has shown that lateralization of the sensorimotor area function in response to passive motor stimulation of the knee and elbow joint can be imaged and evaluated even in newborn infants during sleep under sedation. Data obtained using MNIRS should be useful for understanding the pathophysiology of the developing regional brain and the brain's response to damage in various clinical situations.
Abbreviations
- deoxyHb:
-
deoxyhemoglobin
- fMRI:
-
functional magnetic resonance imaging
- MNIRS:
-
multichannel near-infrared spectroscopy
- NIRS:
-
near-infrared spectroscopy
- oxyHb:
-
oxyhemoglobin
- totalHb:
-
total hemoglobin
REFERENCES
Isobe K, Kusaka T, Nagano K, Okubo K, Yasuda S, Kondo M, Itoh S, Onishi S 2001 Functional imaging of the brain in sedated newborn infants using near infrared topography during passive knee movement. Neurosci Lett 299: 221–224
Hintz SR, Benaron DA, Siegel AM, Zourabian A, Stevenson DK, Boas DA 2001 Bedside functional imaging of the premature infant brain during passive motor activation. J Perinat Med 29: 335–343
Heep A, Scheef L, Jankowski J, Born M, Zimmermann N, Sival D, Bos A, Gieseke J, Bartmann P, Schild H, Boecker H 2009 Functional magnetic resonance imaging of the sensorimotor system in preterm infants. Pediatrics 123: 294–300
Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C 2002 Relative right versus left frontal EEG in neonates. Dev Psychobiol 41: 147–155
Erberich SG, Panigrahy A, Friedlich P, Seri I, Nelson MD, Gilles F 2006 Somatosensory lateralization in the newborn brain. Neuroimage 29: 155–161
Dehaene-Lambertz G 2000 Cerebral specialization for speech and nonspeech stimuli in infants. J Cogn Neurosci 12: 449–460
Peña M, Maki A, Kovacić D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J 2003 Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci U S A 100: 11702–11705
Meek JH, Firbank M, Elwell CE, Atkinson J, Braddick O, Wyatt JS 1998 Regional hemodynamic responses to visual stimulation in awake infants. Pediatr Res 43: 840–843
Kusaka T, Kawada K, Okubo K, Nagano K, Namba M, Okada H, Imai T, Isobe K, Itoh S 2004 Noninvasive optical imaging in the visual cortex in young infants. Hum Brain Mapp 22: 122–132
Karen T, Morren G, Haensse D, Bauschatz AS, Bucher HU, Wolf M 2008 Hemodynamic response to visual stimulation in newborn infants using functional near-infrared spectroscopy. Hum Brain Mapp 29: 453–460
Sakatani K, Chen S, Lichty W, Zuo H, Wang YP 1999 Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Hum Dev 55: 229–236
Nishida T, Kusaka T, Isobe K, Ijichi S, Okubo K, Iwase T, Kawada K, Namba M, Imai T, Itoh S 2008 Extrauterine environment affects the cortical responses to verbal stimulation in preterm infants. Neurosci Lett 443: 23–26
Bartocci M, Winberg J, Ruggiero C, Bergqvist LL, Serra G, Lagercrantz H 2000 Activation of olfactory cortex in newborn infants after odor stimulation: a functional near-infrared spectroscopy study. Pediatr Res 48: 18–23
Taga G, Asakawa K, Hirasawa K, Konishi Y 2003 Hemodynamic responses to visual stimulation in occipital and frontal cortex of newborn infants: a near-infrared optical topography study. Early Hum Dev 75: S203–S210
Hoshi Y, Kohri S, Matsumoto Y, Cho K, Matsuda T, Okajima S, Fujimoto S 2000 Hemodynamic responses to photic stimulation in neonates. Pediatr Neurol 23: 323–327
Konishi Y, Taga G, Yamada H, Hirasawa K 2002 Functional brain imaging using fMRI and optical topography in infancy. Sleep Med 3: S41–S43
Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H 2003 Brain imaging in awake infants by near-infrared optical topography. Proc Natl Acad Sci U S A 100: 10722–10727
Villringer A, Chance B 1997 Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci 20: 435–442
Colier WN, Quaresima V, Oeseburg B, Ferrari M 1999 Human motor-cortex oxygenation changes induced by cyclic coupled movements of hand and foot. Exp Brain Res 129: 457–461
Obrig H, Hirth C, Junge-Hulsing JG, Doge C, Wolf T, Dirnagl U, Villringer A 1996 Cerebral oxygenation changes in response to motor stimulation. J Appl Physiol 81: 1174–1183
Colonnese MT, Phillips MA, Constantine-Paton M, Kaila K, Jasanoff A 2008 Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci 11: 72–79
Chugani HT, Phelps ME 1986 Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 231: 840–843
Chugani HT, Phelps M, Mazziotta J 1987 Positron emission tomography study of human brain functional development. Ann Neurol 22: 487–497
Kinnala A, Suhonen-Polvi H, Aärimaa T, Kero P, Korvenranta H, Ruotsalainen U, Bergman J, Haaparanta M, Solin O, Nuutila P, Wegelius U 1996 Cerebral metabolic rate for glucose during the first six months of life: an FDG positron emission tomography study. Arch Dis Child Fetal Neonatal Ed 74: F153–F157
Chiron C, Raynaud C, Maziere B, Zilbovicius M, Laflamme L, Masure M, Dulac O, Bourguignon M, Syrota A 1992 Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med 33: 696–703
Takahashi T, Shirane R, Sato S, Yoshimoto T 1999 Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol 20: 917–922
Wintermark M, Lepori D, Cotting J, Roulet E, van Melle G, Meuli R, Maeder P, Regli L, Verdun F, Deonna T, Schnyder P, Gudinchet F 2004 Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics 113: 1642–1652
Eyre JA, Miller S, Clowry GJ, Conway EA, Watts C 2000 Functional corticospinal projections are established prenatally in the human foetus permitting involvement in the development of spinal motor centres. Brain 123: 51–64
Erberich SG, Friedlich P, Seri I, Nelson MD Jr, Bluml S 2003 Functional MRI in neonates using neonatal head coil and MR compatible incubator. Neuroimage 20: 683–692
Author information
Authors and Affiliations
Additional information
Supported by grants-in-aid for scientific research (C) no. 20591299, 22591202, 22591201, 22591203, and 17390307 from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Sanju Alumni Research Grant 21-1; and The Mother and Child Health Foundation.
Rights and permissions
About this article
Cite this article
Kusaka, T., Isobe, K., Miki, T. et al. Functional Lateralization of Sensorimotor Cortex in Infants Measured Using Multichannel Near-Infrared Spectroscopy. Pediatr Res 69, 430–435 (2011). https://doi.org/10.1203/PDR.0b013e3182125cbd
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3182125cbd
This article is cited by
-
A Review of Functional Near-Infrared Spectroscopy Studies of Motor and Cognitive Function in Preterm Infants
Neuroscience Bulletin (2020)