Abstract
Background:
Maternal thyroid autoantibodies during pregnancy have been implicated in neurodevelopmental delays, including early childhood cognitive deficits. We evaluated whether maternal autoantibodies to thyroid peroxidase (TPOaAbs) during late pregnancy were associated with childhood intelligence quotient (IQ) scores in their offspring and how the children’s TPOaAb-associated sensorineural hearing loss (HL) might affect the result.
Methods:
We evaluated banked third-trimester sera corresponding to 1,733 children for whom childhood cognitive test scores and audiology data were available. The mothers and their children participated in the National Institutes of Health (NIH)-sponsored Collaborative Perinatal Project (CPP) that ran from 1959 to 1974.
Results:
A modest, statistically significant, effect of TPOaAbs on cognitive performance observed at 4 y of age lessened in both magnitude and P value by the age of 7 y. Children with sensorineural HL (SNHL) had lower IQ scores at both ages.
Conclusion:
Our data suggest that the reported effect of maternal TPOaAbs on IQ may involve early developmental delays or transient effects rather than permanent deficits. Reports associating TPOaAbs directly with IQ may reflect a portion with unexamined TPOaAb-associated SNHL. Whether the TPOaAb-associated SNHL is in the neurodevelopmental pathway of later cognitive delays or is independently associated with IQ requires investigation in other studies.
Similar content being viewed by others
Main
Childhood intelligence quotient (IQ) scores and their determinants are generally considered predictors of eventual school performance, quality of life, and psychiatric morbidity (1,2). The value of inferences and interpretations of IQ scores has been debated for decades, and earlier beliefs that they signal an innate and heritable ability have been largely modified in recognition of the social and economic conditions they reflect. Nonetheless, the lower end of the score range is a useful metric (along with other assessments), especially where handicaps are concerned, to identify children and their parents who are in need of specialized support and educational services.
In regions with iodine-poor diets, cognitive deficits associated with thyroid dysfunction in mothers and neonates have been recognized for more than a century. It has also been observed repeatedly that, aside from dietary iodine insufficiency, prolonged elevation of thyroid autoantibodies—especially those to thyroid peroxidase (TPO)—may lead to a gamut of neurodevelopmental delays, including cognitive deficits (3,4,5,6,7,8,9). The presence of circulating thyroid autoantibodies does not necessarily presage clinical disease, and affordable technological advances are changing our understanding of the prevalence and possible implications of subclinical autoantibody elevation (10). What proportion of maternal hypothyroxinemia during pregnancy can be attributed to elevated TPO autoantibodies (TPOaAbs), for instance, has not been established, nor whether elevated TPOaAbs necessarily reduce thyroxine availability to the developing fetal brain. Nonetheless, several studies of pregnant women with elevated TPOaAbs found that their children had impaired intellectual performance whether or not the mothers also had experienced clinical thyroid dysfunction (11,12,13). Investigators from one study reported that children of women with elevated TPOaAbs but normal thyroid function during late gestation scored 10.5 points lower on the McCarthy scale (a proxy for IQ) than those born to women who were TPOaAb-negative (13).
This study used banked sera from the Baltimore site of the Collaborative Perinatal Project (CPP) of the National Institutes of Health (NIH)/National Institute of Neurological Diseases and Stroke. One of the primary objectives of that longitudinal study, conducted from 1959 to 1974, was to identify perinatal events associated with intelligence outcomes in the offspring.
We previously observed that TPOaAb elevation in women during the third trimester of pregnancy was strongly (prevalence odds ratio 7.5) and significantly (95% confidence interval: 2.4, 23.3) associated with sensorineural hearing loss (SNHL) in their children (14). The strength of the association became more robust as the TPOaAb concentration increased, even in women who were clinically euthyroid. In light of the literature concerning TPOaAbs and cognitive function and our findings regarding TPOaAbs and SNHL, we questioned whether the children’s SNHL status might partially explain the lower IQ scores of children exposed to maternal TPOaAbs, i.e., whether SNHL had acted as an unmeasured confounder in other TPOaAb–IQ study findings, or cognitive function and hearing loss (HL) might share a pathogenic pathway associated with TPOaAbs.
Results
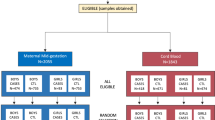
Third-trimester maternal sera were available for 1,527 mothers of 1,733 children who met the inclusion criteria. Black children made up 82.4% of the study population and white children comprised the other 17.6%. The mean IQ score at the age of 7 y was 92 (±12). Black mothers were a year older than white mothers (24.3 vs. 23.3) and had nearly a year more of schooling (9.8 vs. 9.0 y; P = 0.001). The black fathers also had somewhat more schooling than their white counterparts (9.9 vs. 9.1 y; P = 0.001). There was no difference between the self-designated racial groups in the distribution of TPOaAbs or in the proportion coded as positive for parental mental retardation.
Maternal TPOaAb and IQ Scores
In unadjusted regression analysis, the (log) continuous increase in TPOaAb IU/ml was inversely associated with IQ scores on the Stanford–Binet at the age of 4 y and again on the full-scale Wechsler scores at the age of 7 y ( Table 1 ). Whereas the effect of TPOaAbs was statistically significant (P = 0.04) in the preschool group, both the magnitude and the P value had attenuated among 7 y olds, even when entered in a mutually adjusted model (P = 0.08).
Childhood IQ Scores and SNHL
Audiology results classified 131 children (7.5% prevalence) as meeting the pediatric case definition of SNHL. In unadjusted assessments, SNHL was strongly associated with IQ scores ( Table 2 ; data from 4 y olds not shown).
Other Cofactors in Association With IQ Scores
Numerous maternal and child cofactors potentially associated with cognitive scores were evaluated for an independent association with the results on the IQ tests. Higher parental education and white race were significant predictors of higher scores. Maternal age during pregnancy was not independently associated with the IQ of the child but was included because of its association with TPOaAbs and years of maternal education. Several potential cofactors screened, such as prematurity, respiratory distress, and maternal smoking history, which might be expected to have a significant bearing on IQ scores, did not (P > 0.3, data not shown). The CPP protocol included evaluating pregnant participants for thyroid disease and using levothyroxine for the treatment of those with clinical hypothyroidism (N = 23). Despite the small number of women involved, the variable denoting clinical diagnosis and treatment for hypothyroidism, therefore, was treated as a potential confounder and included as an obligatory cofactor in model assessments.
In the final, mutually adjusted model to assess predictors of IQ at the age of 7 y, the cofactors associated significantly with the full-scale Wechsler score were birth weight, maternal education, white race, and SNHL ( Table 3 ). Maternal TPOaAbs, which had been clearly associated with lower IQ scores at the age of 4 y (β = −0.98; P = 0.03), were less strongly and less significantly associated in the multivariate model for the age of 7 y ( Table 3 ). After removal of SNHL from the multivariate model, the effect size of maternal TPOaAbs increased slightly (β = −0.64), and the P value gained marginally in significance (P = 0.07). When the TPOaAb variable was removed, the effect size of SNHL rose modestly, and the P value increased slightly in statistical significance (data not shown). A test for statistical interaction of the TPOaAb and SNHL terms was not significant (P = 0.53).
We conclude from our data that the reported association between maternal TPOaAbs and cognitive deficits in children may be mediated by the effect of maternal thyroid autoantibodies during pregnancy on HL in the offspring.
Discussion
We evaluated whether IQ scores in school-aged children were associated with the presence of TPOaAbs in the sera of their mothers during the third trimester of pregnancy. A total of 1,733 Baltimore children, whose mothers had enrolled in the CPP, were administered the Wechsler Intelligence Scale for Children when they were 7 y old. Of these, 1,647 children had also been evaluated by the Stanford–Binet Scale at the age of 4 y.
We focused this analysis on the results at the longer-term follow-up when the children were 7 y old and the data were more complete. The children underwent complete audiology evaluations when they were 8 y old. Their SNHL status, which we previously observed to be associated with TPOaAbs, was also evaluated with respect to IQ.
In the final multivariate model, maternal TPOaAbs had a small effect on the IQ of the children (β = −0.59), which was of marginal statistical significance (P = 0.08). After screening other sociodemographic and biological cofactors and comparing adjusted models, those robustly associated with the children’s IQ scores were birth weight, white race, and maternal education (positive association) and SNHL (inverse association).
National Health and Nutrition Survey data on TPOaAbs reported a marked difference in prevalence among women of different races. In this study, the distribution of maternal TPOaAbs did not differ by race. Other investigators observed a similar lack of association between race and TPOaAbs during pregnancy (15). We believe that this is because of the decline in autoantibodies during pregnancy, and not an error or bias. Both cognitive performance measured by the Wechsler scale and SNHL were evaluated with respect to the presence of maternal autoantibodies during pregnancy. It is not likely that a sufficient number of cases of SNHL—predominantly a congenital condition—would have occurred during the 12 mo that elapsed between the IQ assessment and the audiology examinations to alter the analytic results.
Secular increases in the mean IQ score nationwide suggest that the Wechsler Intelligence Scale for Children instrument is subject to cultural and environmental influences. The mean IQ in this study group of children was 92 (±12), 8 points below the expected average. This could reflect a bias in the study: selective participation of lower-than-average IQ participants or greater loss to follow-up of the ones who would have scored higher. It could also reflect the situation in Baltimore at the time, including the background presence of unexamined risk factors, such as nutritional deficits or exposure to environmental hazards. Lead exposure was a public health concern in Baltimore during the 1960s and 1970s and even blood lead concentrations of <10 µg/dl have been implicated in poorer cognitive performance (16). Nevertheless, the mean IQ in this study is consistent with averages observed in a study of nationwide CPP data (17,18).
We did not observe an effect of maternal smoking during pregnancy on the intelligence scores of the children. Although this was unexpected, it is not the first time such results have been reported (19). The CPP variable for excessive maternal alcohol intake was uninformative.
Even slight to mild HL (<30 db) has been documented to have life-long effects. Among children with losses <15 dB HL, i.e., whose hearing is considered normal, some research has found that slight fluctuations in auditory perception may affect neurocognitive outcomes (20). Yet these data are relatively recent and scientific opinions are not unanimous.
At least half of all childhood HL remains etiologically unexplained. Epidemiologic studies of childhood HL are scarce and vary considerably in case definitions adopted. One recent review of the literature noted that it was the first in 20 y to systematically consolidate and assess data available on the epidemiology of HL (21). It is hence not surprising that slight to mild impairment would go undetected in many neurodevelopmental studies of children or that children with hearing loss–related delayed language development would score lower on language-dependent cognitive assessments. In our study population, those with SNHL had an HL across the four tested frequencies that was slight to moderate (mean 22 dB HL, range 6–60 dB HL).
Previous studies by other researchers have found an association between TPOaAb elevation in euthyroid women during pregnancy and a reduction in cognitive function among the children. This is postulated to occur as a result of insufficient maternal free thyroxine reserves even when the woman’s thyroid function tests are normal in early pregnancy. Such findings have important population health, clinical screening, and treatment implications. As reported here, we found an effect of third-trimester TPOaAbs on IQ scores in early childhood (at the age of 4 y), but the effect was attenuated in both magnitude and P value among 7 y olds. In their seminal 1995 paper, Pop et al. (13) foresaw the possibility that the early effect might wane with age. Whether our data on 7-y-old children reflect delayed maturation, long-term subtle effects, the possible effects of schooling and structured socialization, fewer missing data for the older children, or some other factor is unknown. Our study looked at pregnant women and their children 50 y ago. Euthyroid pregnant women today still have autoantibodies to TPO and even when thyroid function tests are normal, the children of these women have a higher risk of dying in utero, being born preterm, and suffering adverse outcomes that are only now being elucidated (22). Whether the effects (such as those on SNHL) are the result of subtle fluctuations in maternal thyroxine, some direct insult to the fetus, or a more complex pathology remains unclear. Whether autoantibody-positive euthyroid women should be treated with L-thyroxine during pregnancy is a scientific debate that remains current.
We sought to examine whether the persistent elevation of autoantibodies in late pregnancy was associated with selected neurodevelopmental outcomes. We included the CPP variable for treatment with L-thyroxine as an obligatory cofactor to control for potential confounding by maternal hypothyroxinemia. When the treated women were excluded from the analysis, the results did not change. It is possible that the persistent elevation of maternal TPOaAbs, as detected in third-trimester specimens, is a proxy for subclinical hypothyroxinemia that was not identified or treated by the CPP clinicians despite their rigorous protocol. The association between subclinical hypothyroxinemia during pregnancy and impaired intellectual development in the offspring has been documented (23). Nonetheless, evidence is accumulating that even in euthyroid women, TPOaAbs compromise fetal development (24).
Our findings suggest that even when the TPOaAb levels do not reach clinically significant elevations they may affect neurodevelopment. We conclude that a portion of the cognitive effects in the offspring of mothers with elevated TPOaAbs reported in other studies may have been due to undetected TPOaAb-associated sensorineural HL. The results of this investigation are subtle and would benefit from corroboration in a larger study. However, they are consistent with our earlier finding of a strong association of TPOaAbs with SNHL and the literature on the effects of even mild SNHL on cognitive performance. Rather than contradicting other reports on the observed neurocognitive outcomes of maternal thyroid immunity during pregnancy, therefore, these findings suggest one possible pathway for the association.
The directionality of the association between HL and IQ could be reversed, i.e., children with lower cognitive skills could have performed more poorly on the audiology examinations despite investigator care to ensure that instructions were understood. The strong association observed between elevated maternal TPOaAbs and childhood SNHL, and the lack of an association between mental retardation (IQ < 85, data not shown) and SNHL would argue against such a reversed inference.
TPOaAb titers reportedly decline by at least 50% from the first to the last trimester (25,26). We examined third-trimester sera because we were interested in evaluating associations with persistent autoantibodies. Most of the cognitive effects of maternal TPOaAb positivity reported in the literature refer to children whose mothers were tested earlier in pregnancy. It is possible that some of the data we analyzed were biologically “misclassified” by differences in titer decline, i.e., some sera with low or negligible TPOaAbs may have declined more steeply from higher initial titers than others, weakening our discernment of an association that would have been stronger in an earlier developmental window.
In sum, our findings support evidence that even mild SNHL is associated with subtle but significant effects on IQ. They also suggest that a portion of the reported effects of maternal thyroid TPOaAbs on cognitive development of the children may be associated with another neurodevelopmental outcome associated with TPOaAb—sensorineural HL. This result would have to be confirmed in further studies.
Methods
The study was reviewed and approved by the Johns Hopkins Medical Institutions (JHMI) Institutional Review Board and the Johns Hopkins School of Public Health’s Committee on Human Research and HIPAA. Informed consent was obtained from participants at registration. The JHMI Stanley Division of Developmental Neurovirology provided banked maternal serum specimens from the last half of pregnancy that had been obtained from the repository at the National Institute of Child Health and Development/NIH. The banked sera were originally drawn from pregnant participants at the Baltimore site of the Collaborative Study on Cerebral Palsy, Mental Retardation and Other Neurological and Sensory Disorders of Infancy and Childhood, an observational cohort known as the CPP. Established by the National Institute of Neurological and Communicative Disorders and Stroke of the NIH, 12 sites participated in the CPP, which is described in detail elsewhere (27,28). The NIH-funded Baltimore CPP Partnership maintains a database for research purposes and provided cofactor data, also stripped of individual identifiers. Sample aliquots were coded to mask individual identities, and the TPOaAb assays were conducted before linkage to IQ or SNHL case status. To be included in this study, children were required to have both a full-scale Wechsler score at the age of 7 y and complete audiology examination at the age of 8 y.
Laboratory Methods
The specimens were diluted to a 1:5 ratio in phosphate-buffered saline and analyzed for seropositivity to selected infectious agents by solid-phase enzyme immunoassay as described elsewhere (29). They were then refrozen and stored at −20 °C. We received 50 μl of the remaining aliquots, stripped of individual identifiers. The Stanley Division of Developmental Neurovirology provided a spreadsheet with the linkage to identifying numbers once the TPOaAb assays had been conducted. TPOaAb concentrations were assessed at the JHMI Autoimmune Disease Research Center using an indirect microplate enzyme-linked immunoabsorbent assay (QUANTA Lite TPO, INOVA Diagnostics, San Diego, CA) (30). We logn-transformed the concentrations per the instruction manual to analyze the effects of increments in TPOaAb levels along a continuous scale.
The frozen samples were thawed and diluted by adding 5 μl of serum to 500 μl of horseradish peroxidase diluent provided in the kits. One hundred micoliters of the diluted sample was added to microwells that were precoated with purified human TPO antigen provided by the manufacturer. After incubating for 30 min, the samples were washed three times in diluted horseradish peroxidase (wash concentrate had been provided in the kit), the plates were dried, and 100 μl of horseradish peroxidase IgG conjugate was added, and after three additional washings the samples were incubated for another 30 min. Finally, 100 μl of 3,3′,5,5′ tetramethylbenzidine chromogen was added and the plates were incubated in the dark for another 30 min. Stop solution was added, and the optical densities were read using a Dynex MRX Revelation 4.2 reader (Dynex Technologies, Chantilly, VA). Calibration and control samples for each plate were referenced to the World Health Organization standard (International Reference Preparation MRC 66/387).
Cognitive Assessment
Age-specific scoring systems were administered to test CPP children at the age of 4 y (Stanford–Binet Intelligence Scale) and again at the age of 7 y (Wechsler Intelligence Scale for Children). IQ is the ratio of the mental age divided by chronological age, multiplied by 100. The tests have a mean of 100 and standard deviations of 15 and 16, for the older and younger children, respectively. The Wechsler scale has been in use since the mid-1900s, with modifications to reflect secular changes. We analyzed both test data and report the detailed findings for the school-aged 7-y-olds, for whom the follow-up was the longest and there were fewer missing data.
HL Ascertainment
The audiology evaluation was performed at the final CPP follow-up, when the children were 8 y old. The assessment relied on pure-tone air conduction and bone conduction tests, methods that remain current today. The right ear was tested first if the child’s birthday was an even number, and if odd, the left ear was tested first. The opposite ear was masked when the difference between the ears was ≥40 dB, and quality control was monitored closely. We defined SNHL as an air conduction threshold of ≥20 dB and an air–bone gap of <10 dB at 500, 1,000, 2,000, or 4,000 Hz in either ear; or a bone conduction threshold of ≥20 dB at the same frequencies in either ear. This case definition includes mixed HL, of which SNHL is a component. We also used the more sensitive pediatric case definition (bone conduction threshold of ≥15 dB instead of ≥20 dB), the results of which are reported here.
Statistical Analysis
TPOaAb elevation was modeled as a continuous variable (the logn of the optical density concentrations). IQ scores were evaluated on a continuous scale. SNHL status was modeled as a continuous, categorical, and as the binary variable (meeting each case definition or not) reported here. The association of IQ scores with TPOaAb elevation and SNHL was assessed using the continuous IQ measure, first in unadjusted comparison of means (Student’s t-test) for binary cofactors and linear regression to evaluate continuous variables, and subsequently in multivariate linear regression including cofactors found to be significant after initial screening (Stata v9.0 software; Stata, College Station, TX). Multivariate analyses used methods for robust SEs to account for within-family correlated data arising from same-mother siblings included in the analysis.
Maternal cofactors evaluated for an independent association of IQ scores with TPOaAb elevation and SNHL status included maternal age, race, years of education, history of syphilis, thyroid disease, smoking history, reproductive and medical history (including eclampsia, prior miscarriages, stillbirths, and premature deliveries), and socioeconomic status. An interaction term for TPOaAb level and SNHL with regard to IQ was tested. In addition, CPP binary variables for maternal and paternal mental retardation were screened. Maternal IQ had been measured at the 4-y CPP follow-up visit. A description of the test timing and procedures for the fathers’ mental retardation could not be found, and some 7% of the observations were missing, as were 3.8% of the data for the mother’s cognitive status. As a result, the variables were excluded after exploratory analyses. Maternal IgG antibody levels to Herpes simplex viruses Type 1 and 2, Epstein-Barr virus, cytomegalovirus, and Toxoplasma gondii infections were also evaluated.
Congenital, neonatal, and childhood factors were first evaluated for independent associations with TPOaAb levels, IQ, and/or SNHL. They included such variables as gestational age at the time of the blood draw (gestational age at birth minus the number of days before the delivery that the sample was drawn), bilirubin levels, respiratory distress, oxygen therapy, birth weight and low birth weight (<2,500 g), gestational age at the time of birth (31,32) (days from registration to birth, plus gestational age at registration), CPP variables for head trauma, lead, kerosene, and other toxicities and infections (mumps, measles, and rubella). The imprecision of gestational age estimates is discussed elsewhere (33). We used the CPP gestational age recorded at registration rather than at the last menstrual period, which was also available, because the former had been reviewed by a clinician and, mostly, adjusted for erroneous recall.
Power calculations were conducted before the study began based on the estimated number of specimens available and prevalence rate data. The study was well powered (>80%, two-tailed) to analyze the primary associations, but the study sample was not large enough to conduct robust subgroup or statistical interaction analyses.
Statement of Financial Support
The National Institute of Child Health and Human Development/National Institutes of Health (NIH) repository provided serum specimens to the Stanley Division of Developmental Neurovirology, Johns Hopkins Medical Institutions (JHMI), from which samples were obtained. The Stanley Medical Research Institute provided financial support to procure the samples from the NIH repository. The National Institute of Mental Health/NIH supported CPP database maintenance (“Pilot Studies for Baltimore CPP/Pathways Follow-up,” grant MH 070333). INOVA Diagnostics donated the assay kits for the analysis of thyroid autoantibodies. The ELISA tests were supported by the Johns Hopkins Center for Autoimmune Disease Research. The Fetal Physiology Foundation and the Kennedy Krieger Institute/JHMI sponsored E.E.W.’s and J.P.P.’s participation in the Neurodevelopment Workshop, Baltimore, MD, 2008.
References
Snowling MJ, Bishop DV, Stothard SE, Chipchase B, Kaplan C . Psychosocial outcomes at 15 years of children with a preschool history of speech-language impairment. J Child Psychol Psychiatry 2006;47:759–65.
Koenen KC, Moffitt TE, Roberts AL, et al. Childhood IQ and adult mental disorders: a test of the cognitive reserve hypothesis. Am J Psychiatry 2009;166:50–7.
Berbel P, Mestre JL, Santamaría A, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid 2009;19:511–9.
Poppe K, Glinoer D . Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum Reprod Update 2003;9:149–61.
Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55.
Man EB, Brown JF, Serunian SA . Maternal hypothyroxinemia: psychoneurological deficits of progeny. Ann Clin Lab Sci 1991;21:227–39.
Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ . Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8.
Dallas JS . Autoimmune thyroid disease and pregnancy: relevance for the child. Autoimmunity 2003;36:339–50.
Klein RZ, Mitchell ML . Maternal hypothyroidism and cognitive development of the offspring. Curr Opin Pediatr 2002;14:443–6.
Zöphel K, Saller B, Wunderlich G, et al. Autoantibodies to thyroperoxidase (TPOAb) in a large population of euthyroid subjects: implications for the definition of TPOAb reference intervals. Clin Lab 2003;49:591–600.
Muller AF, Drexhage HA, Berghout A . Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev 2001;22:605–30.
Lazarus JH . Thyroid disease in pregnancy and childhood. Minerva Endocrinol 2005;30:71–87.
Pop VJ, de Vries E, van Baar AL, et al. Maternal thyroid peroxidase antibodies during pregnancy: a marker of impaired child development? J Clin Endocrinol Metab 1995;80:3561–6.
Wasserman EE, Nelson K, Rose NR, et al. Maternal thyroid autoantibodies during the third trimester and hearing deficits in children: an epidemiologic assessment. Am J Epidemiol 2008;167:701–10.
Walker JA, Illions EH, Huddleston JF, Smallridge RC . Racial comparisons of thyroid function and autoimmunity during pregnancy and the postpartum period. Obstet Gynecol 2005;106:1365–71.
Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, Bellinger DC . Neuropsychological function in children with blood lead levels <10 microg/dL. Neurotoxicology 2007;28:1170–7.
Broman SH, Nichols PL, Kennedy WA . Preschool IQ Prenatal and Early Developmental Correlates. Hillsdale, NJ: Lawrence Erlbaum Associates, 1975.
Gray KA, Klebanoff MA, Brock JW, et al. In utero exposure to background levels of polychlorinated biphenyls and cognitive functioning among school-age children. Am J Epidemiol 2005;162:17–26.
Batty GD, Der G, Deary IJ . Effect of maternal smoking during pregnancy on offspring’s cognitive ability: empirical evidence for complete confounding in the US national longitudinal survey of youth. Pediatrics 2006;118:943–50.
Welch D, Dawes PJ . Variation in the normal hearing threshold predicts childhood IQ, linguistic, and behavioral outcomes. Pediatr Res 2007;61:737–44.
Mehra S, Eavey RD, Keamy DG Jr . The epidemiology of hearing impairment in the United States: newborns, children, and adolescents. Otolaryngol Head Neck Surg 2009;140:461–72.
Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A . Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ 2011;342:d2616.
Klein RZ, Mitchell ML . Maternal hypothyroidism and child development. A review. Horm Res 1999;52:55–9.
Stagnaro-Green A . Thyroid antibodies and miscarriage: where are we at a generation later? J Thyroid Res 2011;2011:841949.
Glinoer D, Delange F . The potential repercussions of maternal, fetal, and neonatal hypothyroxinemia on the progeny. Thyroid 2000;10:871–87.
Smyth PP, Wijeyaratne CN, Kaluarachi WN, et al. Sequential studies on thyroid antibodies during pregnancy. Thyroid 2005;15:474–7.
LaBenz P, LaBenz E . Early Correlates of Speech, Language and Hearing. Littleton, MA: PSG Publishing, 1980.
Hardy J, Shapiro S . Pathways to Adulthood: A Three-Generation Urban Study, 1960–1994: [Baltimore, MD]. Ann Arbor, MI: Inter-university Consortium for Political and Social Research, 1999.
Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH . Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry 2001;58:1032–7.
QUANTA Lite_ TPO for in vitro diagnostic use [laboratory reference manual]. INOVA Diagnostics, San Diego, CA, 2003.
Nguyen RH, Wilcox AJ . Terms in reproductive and perinatal epidemiology: I. Reproductive terms. J Epidemiol Community Health 2005;59:916–9.
Nguyen RH, Wilcox AJ . Terms in reproductive and perinatal epidemiology: 2. Perinatal terms. J Epidemiol Community Health 2005;59:1019–21.
Shea KM, Wilcox AJ, Little RE . Postterm delivery: a challenge for epidemiologic research. Epidemiology 1998;9:199–204.
Acknowledgements
We thank the late Janet Hardy, one of the original Baltimore CPP investigators, for her early guidance on the CPP; and Frank Lassman, an original CPP investigator, for his clarification and advice regarding methods and data. We thank Gary Raymond, Shawn Duffy, and Bruce White, of the Kennedy Krieger Institute/JHMI Information Systems Department, for their invaluable technical assistance. We thank Bodgana Krivogorsky, Stanley Division of Developmental Neurovirology, for aliquotting and labeling the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wasserman, E., Pillion, J., Duggan, A. et al. Childhood IQ, hearing loss, and maternal thyroid autoimmunity in the Baltimore Collaborative Perinatal Project. Pediatr Res 72, 525–530 (2012). https://doi.org/10.1038/pr.2012.117
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2012.117
This article is cited by
-
TPO antibody in euthyroid pregnant women and cognitive ability in the offspring: a focused review
Journal of Endocrinological Investigation (2022)
-
Inconsistencies in the management of neonates born to mothers with “thyroid diseases”
European Journal of Pediatrics (2018)
-
Maternal Thyroxine Levels During Pregnancy and Outcomes of Cognitive Development in Children
Molecular Neurobiology (2016)