Abstract
Neonatal polymorphonuclear leukocytes (PMN) exhibit delayed apoptosis both constitutively and under inflammatory conditions, and evidence has linked PMN longevity to the presence of antiapoptotic proteins. Activation of the survival-associated transcription factor, nuclear factor kappa B (NF-κB), promotes the synthesis of several antiapoptotic proteins including Flice inhibitory protein (FLIP). Neonatal and adult PMN were compared in this study to test the hypothesis that FLIP modulates age-related apoptosis. Expression of the short isoform, FLIP-S, was prominent at baseline and persisted during spontaneous apoptosis in neonatal PMN, whereas basal expression was lower and decreased under the same conditions in adult PMN. Stable FLIP-S expression in neonatal PMN was associated with a relative resistance to apoptosis in response to the protein synthesis inhibitor, cycloheximide (CHX), or the NF-κB inhibitor, gliotoxin. In contrast, similar treatment of adult PMN promoted greater overall apoptosis accompanied by FLIP degradation. Nuclear levels of phosphorylated p65, a critical NF-κB dimer, were relatively robust in neonatal PMN under basal conditions or after stimulation with TNF-α, a cytokine that induces FLIP. In conclusion, persistent FLIP-S expression is involved in the longevity of neonatal PMN, and our data suggest a contribution of NF-κB signaling and related survival mechanisms.
Similar content being viewed by others
Main
The timely resolution of inflammatory processes is dependent on the efficient clearance of apoptotic neutrophils [polymorphonuclear leukocytes (PMN)] (1), while prolonged PMN survival can contribute to chronic inflammation (2). Neonatal PMN are relatively resistant to both spontaneous and Fas-mediated apoptosis (3,4). In addition, neonatal PMN exhibit marked survival responses to cytokines (3,5) identified with bronchopulmonary dysplasia and other neonatal inflammatory disorders (6,7). Neonatal PMN exhibit diminished functional expression of caspase-3, a critical effector of apoptosis, as well as other key apoptotic proteins (4,8). In addition, neonatal PMN have an impaired ability to alter membrane potential in response to stimulation (9), also observed to contribute to delayed PMN apoptosis during sepsis (10). Neonatal PMN also exhibit a diminished apoptotic response to treatment with the protein synthesis inhibitor, cycloheximide (CHX) (3), consistent with the presence of preformed survival proteins (11). Thus, neonatal PMN are characterized by altered function of death mechanisms that favor survival under basal and inflammatory conditions, although these remain incompletely defined.
Flice inhibitory protein (FLIP) is a prototypical antiapoptotic protein critical to the survival of hematopoietic progenitors, although its role in PMN is less clear (12,13). Synthesis of FLIP and related antiapoptotic proteins is regulated in part by the transcription factor, nuclear factor kappa B (NF-κB) (14–17). Modulation of the NF-κB pathway by inflammatory cytokines such as TNF-α (11,18–20) can induce FLIP (14), including the short isoform of FLIP (FLIP-S) in PMN (13). In addition, Vancurova et al. (21) observed enhanced TNF-α-mediated activation of NF-κB in neonatal PMN. However, the contribution of FLIP expression to the prolonged survival of neonatal PMN has not been described. We designed this study to test the hypothesis that increased FLIP expression in neonatal PMN is a mechanism underlying their relative longevity.
METHODS
Donor characteristics.
Heparinized blood samples from the peripheral veins of healthy adult donors (aged 18–55 y) and from the umbilical venous cord blood of freshly delivered term placentas were processed in parallel. Cord blood donors met strict criteria including term gestation, planned C-section, normal intrauterine growth, and the documented absence of acute, chronic or gestational illness or infection, medications, tobacco and/or recreational drug use, and fetal genetic/structural abnormalities. Samples were not used in the event of low (<5) neonatal Apgar scores. All blood samples were collected from donors following informed consent according to a protocol approved by the Institutional Review Board for Human Studies at St. Louis University.
PMN isolation and culture.
PMN were isolated as described (5). Isolated PMN suspended in RPMI 1640/2% FCS were incubated at 37°C, 5% CO2 for up to 24 h in the presence or absence of specific stimuli or inhibitors.
Reagents.
RPMI 1640 and FCS were purchased from GIBCO-BRL (Invitrogen Corporation, Carlsbad, CA) and Hyclone, Inc. (Logan, UT), respectively. Anti-FLIP and anti-XIAP (X-linked inhibitor of apoptosis) antibodies were purchased from Axxora (San Diego, CA), and antibodies against the nuclear marker PCNA (proliferation cellular nuclear antigen) and secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies against IκBα (I kappa B alpha), IKK (IκB kinase), and the NF-κB subunit, p65, and their phosphorylated forms were purchased from Cell Signaling Technology (Beverly, MA). Recombinant human TNF-α was purchased from R&D Systems (Minneapolis, MN). Anti-β-actin antibodies, gliotoxin, and CHX were purchased from Sigma Chemical Co.-Aldrich, Inc. (St. Louis, MO).
Apoptosis studies.
To correlate de novo FLIP synthesis with survival, PMN were cultured with the protein synthesis inhibitor CHX and treated or control cells were analyzed for apoptosis by TUNEL assay (3). To examine a potential survival effect of constitutive NF-κB activation, PMN were treated with the NF-κB inhibitor, gliotoxin (11,22), and apoptosis was assessed by TUNEL assay. In parallel studies, the apoptotic effects of each treatment were correlated with FLIP expression in PMN lysates, analyzed by Western blot.
TUNEL assay.
A commercial flow cytometric TUNEL assay (In Situ Cell Death Detection Kit; Boehringer Mannheim, Inc., Mannheim, Germany) was used to detect and quantify apoptotic cell death by enzymatic labeling of DNA strand breaks with fluorescein (dUTP) and terminal deoxy-nucleotidyl transferase (TdT), as described (3).
Western blots.
For whole cell studies, PMN (5 × 106) were lysed in RIPA buffer containing a protease inhibitor cocktail (both, Sigma Chemical Co.-Aldrich). Nuclear fractions were prepared from PMN (107) stimulated with TNF-α, using the NE-PER kit (Pierce, Rockford, IL). For phosphoprotein expression, the lysis buffer was supplemented with 2 mM each of Na orthovanadate and Na fluoride. Equivalent protein amounts of lysates in 2× Laemmli buffer were separated by SDS-PAGE and analyzed by Western blot using primary antibodies and horseradish peroxidase-conjugated secondary antibodies. Protein sample loading was normalized by reprobing blots with anti-β-actin antibodies or with a nuclear marker, as described (23,24). Protein bands were visualized by chemiluminescence (ECL), and band intensities relative to β-actin or PCNA expression or the ratio of phosphorylated to total cognate protein were quantified by densitometric analysis.
Statistical analysis.
Data expressed as mean ± SD were analyzed by t test or by ANOVA, as appropriate, using a statistical software program (Sigma Chemical Co. Stat for Windows, SPSS, Inc.). A two-way ANOVA with a fixed factor effect was performed where appropriate to assess differences between neonatal (CB) and adult (AD) groups across time. All analyses used an α-level 0.05 to determine statistical significance.
RESULTS
FLIP expression during spontaneous apoptosis.
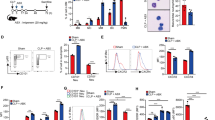
Expression levels of FLIP were determined in lysates of PMN at baseline (0 h) and during spontaneous apoptosis (24 h culture) (3,11). Basal expression of FLIP-S was greater than that of the long form (FLIP-L) and expression of each decreased relative to basal levels during spontaneous apoptosis in both neonatal and adult PMN (Fig. 1A). However, FLIP-S expression was more prominent in neonatal PMN under both basal and apoptotic conditions. Expression of another antiapoptotic protein, XIAP, was similar at 0 or 24 h (Fig. 1B), confirming previous observations (13), and expression did not differ between groups.
FLIP expression in neonatal and adult PMN. FLIP isoforms and β-actin proteins were detected by Western blot in lysates of adult (AD) and neonatal (CB) PMN (PMN) cultured for 0–24 h. Lysates of PMN harvested at each time point were prepared and blotted with monoclonal anti-FLIP or anti-XIAP antibodies. The blots shown are representative of five (A) or two (B) separate paired (AD, CB) experiments.
Differential responses of neonatal and adult PMN to CHX-induced apoptosis and FLIP degradation.
Neutrophils were incubated with CHX to determine a link between increased FLIP expression and de novo protein synthesis. The higher dose of CHX tested (100 μg/mL) induced a greater apoptotic response in adult versus neonatal PMN after incubation for 3 or 6 h, whereas a difference between groups was observed for the lower dose (50 μg/mL) only after the longer incubation (Fig. 2A), confirming our previous observations (3). Diminished apoptosis of neonatal PMN in response to CHX (3 h) (Fig. 2B) was accompanied by persistent expression of FLIP isoforms, with a more prominent expression of FLIP-S (Fig. 2C). In contrast, CHX treatment of adult PMN was associated with decreased expression of both FLIP isoforms (Fig. 2C) that paralleled the greater apoptotic responses of these cells (Fig. 2B). No differences were observed either in the proportion of apoptotic cells or in the expression of FLIP-S compared with respective baseline values in control PMN from either group.
Neonatal and adult PMN apoptosis in response to CHX. (A) PMN isolated from AD (black bars) and CB (gray bars) donors were incubated for up to 6 h in the presence of the protein synthesis inhibitor CHX (50 or 100 μg/mL) or in vehicle alone, and the resultant proportion of apoptotic cells were determined by flow cytometry (TUNEL assay). *p < 0.05, **p < 0.001; AD vs CB PMN. Data, representative of seven paired (AD, CB) separate experiments, were analyzed by univariate two-way ANOVA with a fixed factor effect. (B, C) PMN were harvested at baseline (0 h) or after treatment with CHX (100 μg/mL, 3 h) or vehicle control in three separate, paired experiments. (B) PMN were assessed for apoptosis by TUNEL assay. *p < 0.05, AD vs CB PMN. (C) FLIP and β-actin were detected by Western blot in AD and CB PMN lysates with monoclonal anti-FLIP antibodies.
TNF-α induced activation of the NF-κB pathway in neonatal and adult PMN.
In studies to compare NF-κB activity in neonatal and adult PMN, nuclear expression levels of the cognate and phosphorylated forms of IKK, IκBα, and p65 were determined under basal conditions and after TNF-α treatment (Fig. 3A and B). Transient phosphorylation of IKK peaked at 30 min in neonatal PMN and at 60 min in adult PMN. Prominent phosphorylation of nuclear IκBα in neonatal PMN was observed at baseline and throughout the study interval and was associated with IκBα degradation at 30 min. In contrast, in adult PMN, phosphorylated IκBα levels were most evident at 15 and 30 min of treatment, and IκBα expression levels were lowest at 60 min. The phosphorylation status of nuclear p65/RelA changed significantly from basal values over the treatment period in both neonatal and adult PMN (p < 0.001), whereas levels of the unphosphorylated protein remained similar (Fig. 3A and B). In neonatal PMN, phospho-p65 levels were robust under basal conditions and phosphorylation status decreased over the ensuing 30 min, followed again by prominent expression at 60 min. In contrast, the phosphorylation status of p65 was not as pronounced in adult PMN; phospho-p65 levels were minimal at baseline and levels peaked at 15 min of treatment, decreasing thereafter.
NF-κB activation in neonatal and adult PMN nuclear fractions. PMN treated with TNF-α (10 ng/mL) were harvested at the indicated times. (A) Nuclear fractions were subjected to Western blotting using antibodies specific to IKK, IκBα, and p65 (or their phosphorylated forms). Blots were reprobed with anti-PCNA antibodies as a loading control. (B) Levels of phosphorylated p65 protein were normalized to total cognate protein, and the mean densitometric ratios are shown in the graph. *p < 0.05 and **p < 0.01, AD PMN (black line) vs CB PMN (gray line). Blot shown is representative of three paired (AD, CB), separate experiments.
Differential gliotoxin effect on apoptosis and FLIP expression in neonatal and adult PMN.
We next examined whether augmented constitutive activation of NF-κB might underlie the relative resistance of neonatal PMN to spontaneous apoptosis (3). Neonatal and adult PMN were incubated with gliotoxin, a specific NF-κB inhibitor (22), and apoptosis determined by flow cytometry. Gliotoxin treatment induced a peak apoptotic response in neonatal PMN (0.01 μg/mL, 49 ± 18%) at a 10-fold lower dose than the higher peak response in adult PMN (0.1 μg/mL, 76 ± 10%; p < 0.001; Fig. 4A). As FLIP expression is known to be regulated by NF-κB (14), we examined the effect of gliotoxin treatment on FLIP expression in conjunction with apoptosis. FLIP expression remained prominent in treated neonatal PMN, whereas it decreased in adult PMN (Fig. 4B). FLIP-S expression was greater than that of FLIP-L, as previously reported (13). Persistence of FLIP-S expression in gliotoxin-treated neonatal PMN paralleled relatively diminished apoptotic responses (Fig. 4C), whereas diminished levels of FLIP-S corresponded to increased apoptosis in adult PMN.
Sensitivity of neonatal and adult PMN to gliotoxin-induced apoptosis. (A) Neonatal (CB) and adult (AD) PMN were incubated for 6 h with increasing concentrations of gliotoxin, a specific NF-κB inhibitor. The proportions of apoptotic PMN in gliotoxin-treated cultures relative to apoptotic PMN in control cultures were determined by flow cytometry (TUNEL assay). The percentages of apoptotic PMN in CB cultures (gray line) peaked at a lower gliotoxin dose, and peak levels were lower than for AD PMN (black line; *p < 0.001). Data represent the mean values of eight separate, paired (AD, CB) experiments. To correlate FLIP expression with apoptosis, PMN were incubated for 3 h with gliotoxin (0.1 μg/mL); PMN lysates or intact PMN were assessed by Western blot (B) or flow cytometry (C), respectively. (B) FLIP and β-actin proteins were detected by Western blot in PMN lysates. Blot shown is representative of three paired (AD, CB), separate experiments. (C) Apoptotic fractions in AD (black bars) and CB (gray bars) PMN were determined by TUNEL assay. * p < 0.05, adult vs neonatal PMN. Data represent the means of five separate, paired studies.
DISCUSSION
Studies from our laboratory and of others suggest the existence of preformed survival factors (3) and enhanced NF-κB activation (21,25) in neonatal PMN. These observations led us to hypothesize that the constitutive presence of FLIP, an antiapoptotic protein regulated by NF-κB, might be one mechanism underlying the relative resistance of neonatal PMN to spontaneous apoptosis (3,4). FLIP is a critical negative regulator of apoptosis associated with the death receptor complex and caspase activation (26). FLIP exists in two main forms: a prominent longer isoform (FLIP-L) that contains tandem death effector domains (DED) similar to caspase-8, followed by a caspase-like domain, and a shorter isoform, FLIP-S, that possesses only DEDs (27,28). Neutrophils express lower levels of FLIP-L relative to FLIP-S (13), an observation confirmed in this study.
We observed a greater constitutive expression of FLIP-S in neonatal PMN relative to those of adults. Diminished FLIP expression was paralleled by a greater fraction of apoptotic adult PMN, as previously reported (13). In contrast, the persistence of FLIP-S in neonatal PMN during spontaneous apoptosis is consistent with their protracted lifespan (3,4). Persistent FLIP expression has been shown to modulate the resistance of inflammatory macrophages to Fas-mediated apoptosis in patients with rheumatoid arthritis (29,30), and neonatal PMN are also relatively resistant to apoptosis mediated by Fas (3,4). These observations suggest that augmented FLIP expression may directly promote survival in neonatal PMN, although the exact mechanism remains to be discerned. FLIP-S has been shown to inhibit caspase-8/-3 and consequently the intrinsic pathway of apoptosis, particularly in PMN (27,28). Neonatal PMN have blunted activity of these caspases (4,8), which suggests that FLIP may modulate their survival by blocking downstream apoptotic signaling (27) and is a premise that warrants further investigation.
Neonatal PMN persistently expressed FLIP-S protein despite inhibition of de novo synthesis (Fig. 2C), consistent with the presence of preformed protein (11). In contrast, FLIP expression was diminished in similarly treated adult PMN, as previously reported in several cell lines, including HL-60 cells (31,32). Neutrophils exhibit constitutive NF-κB signaling (33,34), which can also induce FLIP synthesis (14,35). This evidence led us to examine a potential contribution of NF-κB to the prominent basal expression of FLIP-S in neonatal PMN. NF-κB signaling in PMN involves nuclear IKK activation, phosphorylation/degradation of IκBα and p65/RelA phosphorylation (23,35,36). In this study, neonatal PMN exhibited earlier IκBα degradation and prominent basal phosphorylation levels of p65/RelA (Fig. 3), consistent with NF-κB activation status (23,35–38). NF-κB signaling is important for constitutive IL-8 promoter activity and IL-8 synthesis (34,39), as well as Akt phosphorylation (40). Pertinently, both IL-8 release and Akt activation status are enhanced under basal conditions in neonatal PMN (5,25), providing further evidence of augmented constitutive NF-κB activation in neonatal PMN. Akt activation may also be involved in the persistence of FLIP expression by inducing a stabilizing protein that blocks FLIP ubiquitination (41,42), a process that results in proteasomal degradation (43). Although ubiquitination of FLIP has not been reported in PMN, our results could be consistent with the existence of such a protein in neonatal PMN, a mechanism with implications for their prolonged survival and inflammatory potential (44).
Studies conducted to correlate basal NF-κB activity with survival function showed a greater sensitivity of neonatal PMN to the apoptotic effects of low-dose gliotoxin under basal conditions, also consistent with enhanced constitutive NF-κB activity. Unexpectedly, however, higher doses of gliotoxin induced a greater overall apoptotic response in adult PMN. One possible explanation for these observations could be that inhibition of NF-κB activity in adult PMN unmasked proapoptotic signals, such as caspases and/or cJun N-terminal kinase (13,45,46). This possibility is strengthened by the relative prominence of caspases and other proapoptotic proteins in adult PMN (4,8). Conversely, the muted apoptotic effect of gliotoxin on neonatal PMN could reflect a predominance of survival signaling mechanisms. Inhibition of NF-κB was shown to promote PMN survival mediated by p38-MAPK (47), although whether p38-MAPK signaling is altered in neonatal PMN is unclear (4,48). Alternatively, robust basal Akt activation status in neonatal PMN (5) suggests that prominence of this survival pathway may be a plausible explanation for our observations. This premise is reinforced by findings that PMN survival may be more dependent on Akt than on NF-κB activation mechanisms (49). Furthermore, Akt potently induces FLIP expression and promotes its stability (41,50), which also suggests its contribution to the persistent FLIP expression in neonatal PMN observed in this study. However, further investigation, beyond the scope of this article, will be required to dissect these potential mechanisms.
In summary, this report establishes a novel association between enhanced FLIP-S expression and the preferential survival of neonatal PMN. In addition, our data suggest a link between FLIP expression and a prominence of NF-κB and related survival signaling pathways. Although the contributory mechanisms remain unclear, age-related differences in FLIP expression are likely important to augmented PMN inflammatory potential. Thus, therapeutic targeting of FLIP or related upstream pathways may be particularly relevant to inflammation in the developing human.
Abbreviations
- AD:
-
adult
- CB:
-
cord blood
- CHX:
-
cycloheximide
- FLIP:
-
Flice inhibitory protein
- FLIP-S:
-
short isoform of FLIP
- FLIP-L:
-
long isoform of FLIP
- IκBα:
-
inhibitor kappa B alpha
- IKK:
-
IκB kinase
- NF-κB:
-
nuclear factor kappa B
- PCNA:
-
proliferation cellular nuclear antigen
- PMN:
-
polymorphonuclear leukocytes
- XIAP:
-
X-linked inhibitor of apoptosis
References
Walker A, Ward C, Taylor EL, Dransfield I, Hart SP, Haslett C, Rossi AG 2005 Regulation of neutrophil apoptosis and removal of apoptotic cells. Curr Drug Targets Inflamm Allergy 4: 447–454
Matute-Bello G, Martin TR 2003 Science review: apoptosis in acute lung injury. Crit Care 7: 355–358
Allgaier B, Shi M, Luo D, Koenig JM 1998 Spontaneous and Fas-mediated apoptosis are diminished in umbilical cord blood neutrophils compared with adult neutrophils. J Leukoc Biol 64: 331–336
Hanna N, Vasquez P, Pham P, Heck DE, Laskin JD, Laskin DL, Weinberger B 2005 Mechanisms underlying reduced apoptosis in neonatal neutrophils. Pediatr Res 57: 56–62
Rashmi R, Bode BP, Panesar N, King SB, Rudloff JR, Gartner MR, Koenig JM 2009 Siglec-9 and SHP-1 are differentially expressed in neonatal and adult neutrophils. Pediatr Res 66: 266–271
Kotecha S, Mildner RJ, Prince LR, Vyas JR, Currie AE, Lawson RA, Whyte MK 2003 The role of neutrophil apoptosis in the resolution of acute lung injury in newborn infants. Thorax 58: 961–967
Oei J, Lui K, Wang H, Henry R 2003 Decreased neutrophil apoptosis in tracheal fluids of preterm infants at risk of chronic lung disease. Arch Dis Child Fetal Neonatal Ed 88: F245–F249
Luo D, Schowengerdt KO Jr, Stegner JJ, May WS Jr, Koenig JM 2003 Decreased functional caspase-3 expression in umbilical cord blood neutrophils is linked to delayed apoptosis. Pediatr Res 53: 859–864
Sacchi F, Hill HR 1984 Defective membrane potential changes in neutrophils from human neonates. J Exp Med 160: 1247–1252
Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC 2004 Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med 32: 1460–1469
Ward C, Chilvers ER, Lawson MF, Pryde JG, Fujihara S, Farrow SN, Haslett C, Rossi AG 1999 NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem 274: 4309–4318
Kim H, Whartenby KA, Georgantas RW 3rd, Wingard J, Civin CI 2002 Human CD34+ hematopoietic stem/progenitor cells express high levels of FLIP and are resistant to Fas-mediated apoptosis. Stem Cells 20: 174–182
Kanayama A, Miyamoto Y 2007 Apoptosis triggered by phagocytosis-related oxidative stress through FLIPS down-regulation and JNK activation. J Leukoc Biol 82: 1344–1352
Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J 2001 NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol 21: 5299–5305
Tschopp J, Irmler M, Thome M 1998 Inhibition of fas death signals by FLIPs. Curr Opin Immunol 10: 552–558
Bagnoli M, Canevari S, Mezzanzanica D 2010 Cellular FLICE-inhibitory protein (c-FLIP) signaling: a key regulator of receptor-mediated apoptosis in physiologic context and in cancer. Int J Biochem Cell Biol 42: 210–213
Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J 1998 Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med 188: 211–216
Heyninck K, Beyaert R 2001 Crosstalk between NF-kappaB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol Cell Biol Res Commun 4: 259–265
Cao Z, Tanaka M, Regnier C, Rothe M, Yamit-hezi A, Woronicz JD, Fuentes ME, Durnin MH, Dalrymple SA, Goeddel DV 1999 NF-kappa B activation by tumor necrosis factor and interleukin-1. Cold Spring Harb Symp Quant Biol 64: 473–483
Vancurova I, Miskolci V, Davidson D 2001 NF-kappa B activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cdelta. Correlation to nuclear Ikappa Balpha. J Biol Chem 276: 19746–19752
Vancurova I, Bellani P, Davidson D 2001 Activation of nuclear factor-kappaB and its suppression by dexamethasone in polymorphonuclear leukocytes: newborn versus adult. Pediatr Res 49: 257–262
Pahl HL, Krauss B, Schulze-Osthoff K, Decker T, Traenckner EB, Vogt M, Myers C, Parks T, Warring P, Muhlbacher A, Czernilofsky AP, Baeuerle PA 1996 The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J Exp Med 183: 1829–1840
Castro-Alcaraz S, Miskolci V, Kalasapudi B, Davidson D, Vancurova I 2002 NF-kappa B regulation in human neutrophils by nuclear I kappa B alpha: correlation to apoptosis. J Immunol 169: 3947–3953
Onelli E, Citterio S, O'Connor JE, Levi M, Sgorbati S 1997 Flow cytometry, sorting and immunocharacterization with proliferating cell nuclear antigen of cycling and non-cycling cells in synchronized pea root tips. Planta 202: 188–195
Nguyen CN, Schnulle PM, Chegini N, Luo X, Koenig JM 2010 Neonatal neutrophils with prolonged survival secrete mediators associated with chronic inflammation. Neonatology 98: 341–347
Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J 1997 Inhibition of death receptor signals by cellular FLIP. Nature 388: 190–195
Hyer ML, Samuel T, Reed JC 2006 The FLIP-side of Fas signaling. Clin Cancer Res 12: 5929–5931
Kataoka T 2005 The caspase-8 modulator c-FLIP. Crit Rev Immunol 25: 31–58
Perlman H, Pagliari LJ, Liu H, Koch AE, Haines GK 3rd, Pope RM 2001 Rheumatoid arthritis synovial macrophages express the Fas-associated death domain-like interleukin-1beta-converting enzyme-inhibitory protein and are refractory to Fas-mediated apoptosis. Arthritis Rheum 44: 21–30
Bai S, Liu H, Chen KH, Eksarko P, Perlman H, Moore TL, Pope RM 2004 NF-kappaB-regulated expression of cellular FLIP protects rheumatoid arthritis synovial fibroblasts from tumor necrosis factor alpha-mediated apoptosis. Arthritis Rheum 50: 3844–3855
Kreuz S, Siegmund D, Scheurich P, Wajant H 2001 NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol 21: 3964–3973
Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, Manzoli L, Cocco L, Martelli AM 2003 Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia 17: 379–389
Ear T, Cloutier A, McDonald PP 2005 Constitutive nuclear expression of the I kappa B kinase complex and its activation in human neutrophils. J Immunol 175: 1834–1842
Cloutier A, Guindi C, Larivee P, Dubois CM, Amrani A, McDonald PP 2009 Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J Immunol 182: 563–571
Takada Y, Andreeff M, Aggarwal BB 2005 Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood 106: 641–649
Miskolci V, Rollins J, Vu HY, Ghosh CC, Davidson D, Vancurova I 2007 NFkappaB is persistently activated in continuously stimulated human neutrophils. Mol Med 13: 134–142
Yu L, Simonson OE, Mohamed AJ, Smith CI 2009 NF-kappaB regulates the transcription of protein tyrosine kinase Tec. FEBS J 276: 6714–6724
Wright HL, Chikura B, Bucknall RC, Moots RJ, Edwards SW 2011 Changes in expression of membrane TNF, NF-kappaB activation and neutrophil apoptosis during active and resolved inflammation. Ann Rheum Dis 70: 537–543
Kunsch C, Rosen CA 1993 NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol 13: 6137–6146
Romashkova JA, Makarov SS 1999 NF-kappaB is a target of AKT in anti-apoptotic PDGF signaling. Nature 401: 86–90
Panner A, Crane CA, Weng C, Feletti A, Parsa AT, Pieper RO 2009 A novel PTEN-dependent link to ubiquitination controls FLIPS stability and TRAIL sensitivity in glioblastoma multiforme. Cancer Res 69: 7911–7916
Panner A, Crane CA, Weng C, Feletti A, Fang S, Parsa AT, Pieper RO 2010 Ubiquitin-specific protease 8 links the PTEN-Akt-AIP4 pathway to the control of FLIPS stability and TRAIL sensitivity in glioblastoma multiforme. Cancer Res 70: 5046–5053
Thompson SJ, Loftus LT, Ashley MD, Meller R 2008 Ubiquitin-proteasome system as a modulator of cell fate. Curr Opin Pharmacol 8: 90–95
Koenig JM, Stegner JJ, Schmeck AC, Saxonhouse MA, Kenigsberg LE 2005 Neonatal neutrophils with prolonged survival exhibit enhanced inflammatory and cytotoxic responsiveness. Pediatr Res 57: 424–429
Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A 2001 Inhibition of JNK activation through NF-kappaB target genes. Nature 414: 313–317
Wicovsky A, Muller N, Daryab N, Marienfeld R, Kneitz C, Kavuri S, Leverkus M, Baumann B, Wajant H 2007 Sustained JNK activation in response to tumor necrosis factor is mediated by caspases in a cell type-specific manner. J Biol Chem 282: 2174–2183
Langereis JD, Raaijmakers HA, Ulfman LH, Koenderman L 2010 Abrogation of NF-kappaB signaling in human neutrophils induces neutrophil survival through sustained p38-MAPK activation. J Leukoc Biol 88: 655–664
Yan SR, Byers DM, Bortolussi R 2004 Role of protein tyrosine kinase p53/56lyn in diminished lipopolysaccharide priming of formylmethionylleucyl-phenylalanine-induced superoxide production in human newborn neutrophils. Infect Immun 72: 6455–6462
Sousa LP, Lopes F, Silva DM, Tavares LP, Vieira AT, Rezende BM, Carmo AF, Russo RC, Garcia CC, Bonjardim CA, Alessandri AL, Rossi AG, Pinho V, Teixeira MM 2010 PDE4 inhibition drives resolution of neutrophilic inflammation by inducing apoptosis in a PKA-PI3K/Akt-dependent and NF-kappaB-independent manner. J Leukoc Biol 87: 895–904
Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, Lee BL 2003 Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci 94: 1066–1073
Acknowledgements
We thank the donors, the obstetricians, and the Labor and Delivery staff at SSM St. Mary's Health Center and our research nurse Amy Cooper, RN, for their gracious assistance in providing samples critical for our studies. We also wish to thank Teresa Staten, BS, for her valuable technical contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by NIH/NICHD HD47401 [to J.M.K], the Fleur-de-Lis Foundation [to R.R.], and the Pediatric Research Institute, Department of Pediatrics, St. Louis University [to J.M.K.].
Rights and permissions
About this article
Cite this article
Rashmi, R., Schnulle, P., Maddox, A. et al. Flice Inhibitory Protein Is Associated With the Survival of Neonatal Neutrophils. Pediatr Res 70, 327–331 (2011). https://doi.org/10.1203/PDR.0b013e3182290062
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e3182290062