Abstract
A recent survey found that approximately 4% of very low birth weight infants in Japan were treated with glucocorticoids postnatally for circulatory collapse thought to be caused by late-onset adrenal insufficiency. We identified 11 preterm infants with clinical signs compatible with this diagnosis (hypotension, oliguria, hyponatremia, lung edema, and increased demand for oxygen treatment) and matched them for gestational age with 11 infants without such signs. Blood samples were obtained for cortisol and its precursors from the patient group before the administration of hydrocortisone, and from the control group during the same postnatal week. All samples were analyzed using a gas chromatography-mass spectrometry system. Cortisol concentrations did not differ between the two groups (6.6 ± 4.5 vs 3.4 ± 2.7 μg/dL); however, the total concentration of precursors in the pathway to cortisol production was significantly higher in the patient group (72.2 ± 50.3 vs 25.0 ± 28.5 μg/dL; p < 0.05). We conclude that the clinical picture of late-onset adrenal insufficiency in preterm infants is not a result of an absolute deficiency of cortisol production, but may be a result of a limited ability to synthesize sufficient cortisol for the degree of clinical stress.
Similar content being viewed by others
Main
According to a recent nationwide survey in Japan, about 4% of very low birth weight (VLBW) infants are treated with postnatal steroids because of adrenal insufficiency of prematurity (AOP). This condition was defined in the survey as postnatal steroid usage during a hospital stay for treatment of late-onset circulatory collapse in premature infants (1). Reports on glucocorticoid-responsive hypotension among VLBW infants in the early postnatal period suggest that the hypothalamus-pituitary-adrenal system does not function sufficiently in the immediate postnatal period (2–7), and, as a result, the serum cortisol level is relatively low during the first week of life (8–13). However, it has been suggested that this adrenal insufficiency in preterm infants is transient and that adrenal function tends to return to normal by the end of the second week of life (14). Two randomized control trials have demonstrated the benefit of glucocorticoids for preventing and treating refractory hypotension during the first week of life in VLBW infants (15,16). Therefore, glucocorticoid-responsive hypotension was not considered a common phenomenon beyond the first week of life in this population. However, VLBW infants sometimes develop late-onset glucocorticoid-responsive circulatory collapse.
The purpose of the current study differs from previous reports on the above pathophysiological etiologies, because we focused specifically on late-onset circulatory instability, which is usually prominent after the first week of life in preterm infants. AOP was defined as postnatal steroid treatment for late-onset (after the first week of life) adrenal insufficiency in premature infants. To elucidate the pathogenesis of this disorder, a comparison of steroid hormone concentrations between infants with and without AOP is required. However, measurements of cortisol concentrations in preterm infants suspected to have adrenal insufficiency have been inconsistent, perhaps because of use of different methods for measurement of cortisol in blood samples. Immunoassays for measuring hormone concentrations are sensitive enough to detect small amounts of steroid hormones, but cross-reactivity of antibodies is sometimes a problem. To avoid these problems, HPLC and a gas chromatography-mass spectrometry (GC-MS) assay system were used in this study. A patient-matched control comparative study was performed using the GC-MS assay system to measure steroid hormone concentrations, with the aim of elucidating the pathogenesis of late-onset AOP among preterm infants.
PATIENTS AND METHODS
Patients.
Preterm infants with a gestational age of less than 32 wk without major congenital anomalies and who survived until discharge were eligible for the study. Infants treated with glucocorticoids for any reason before onset of AOP were excluded. The gestational age was determined by an obstetric examination with ultrasonography. Infants weighing less than the 10th percentile of the normal birth weight curve at each gestational age were defined as light-for-date. Antenatal steroid usage was defined as administration of corticosteroids to accelerate fetal lung maturity; infants who received antenatal steroids were not excluded from the study.
Criteria of adrenal insufficiency.
There are no definitive diagnostic criteria for adrenal insufficiency in preterm infants, and therefore independent guidelines are used for glucocorticoid treatment in our neonatal intensive care unit (NICU), with diagnostic criteria determined based on our previous clinical observations. Our analysis indicates that about 30% of infants with at least one of the diagnostic criteria and more than 80% of infants with two of these criteria develop glucocorticoid-responsive severe hypotension within 12 h. Therefore, when more than two of the following five signs are clearly present in a preterm infant at more than 1 wk after birth, a replacement dose of hydrocortisone (1–2 mg/kg/dose) is administered. Adrenal insufficiency is diagnosed when all signs are improved within 6 h after treatment without other supportive treatment.
The five signs of adrenal insufficiency in VLBW infants are hypotension, oliguria, hyponatremia, lung edema, and an increased demand for oxygen treatment. Respiratory instability was included because there is a strong association between adrenal insufficiency and development of chronic lung disease (CLD) in preterm infants (17). Hypotension is defined as a systolic pressure of <40 mm Hg or a 20% reduction from the previous value; oliguria as urine output of <1 mL/kg/h during a 4-h interval; and hyponatremia as a serum sodium concentration of <130 mEq/L or a rapid decrease of >5 mEq/L. Lung edema is diagnosed by chest x-ray examination. Increased oxygen treatment is defined as a rapid increase in the fraction of inspired oxygen by >0.1.
If an infant shows deterioration, including more than two of these signs, then other causes of illness besides AOP, such as infection, anemia, patent ductus arteriosus, and hypovolemia, were first ruled out. Especially, hypovolemia was evaluated based on the total size of the heart on chest x-ray, echographic measurement of the size of the inferior vena cava and the left ventricular diastolic diameter, and the blood flow pattern of peripheral arteries such as the renal and supermesenteric arteries. Thereafter, replacement treatment was administered. Infants who did not respond to this therapy within 6 h were excluded from the study. Infants with early neonatal hypotension (defined as hypotension occurring less than a week after birth) were also excluded.
Sample collection.
Once adrenal insufficiency was suspected according to the diagnostic criteria, 100 μL of blood was obtained before administration of glucocorticoids. Infants without adrenal insufficiency were identified as matched controls in terms of gestational week, within a difference of a week. During a routine check-up, the same amount of blood was collected from controls in the same week after birth as the patients. Finally, if the control infants did not show any signs of adrenal insufficiency during the stay in the NICU, they were formally assigned to the control group. However, potential control infants who were found to be suffering from adrenal insufficiency after blood sampling were included in the patient group and another infant was chosen as a control. If no matched infants could be found for a particular patient, the patient was excluded from the study. The study groups were not masked, but all clinical decisions and interventions were performed by the attending physicians and were basically similar for the two groups. Blood specimens were centrifuged immediately and stored at −70°C until assay.
Assay of steroid hormones.
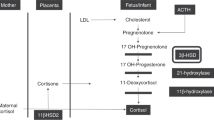
Multiple and simultaneous determination of steroid hormones was carried out as previously reported (18–20). Briefly, standard solutions for pregnenolone, 17-OH-pregnenolone, dehydroepiandrosterone (DHEA), progesterone, 17-OH-progesterone, cortisone, and cortisol were purchased from Steraloids Inc. (Newport, RI). The Oasis Max cartridge (size: 1 mL/30 mg) was used as a solid phase extraction column (Waters Corp., Milford, MA). Arylsulfatase (EC 3.1.6.1, from Helix pomatia) was obtained from Roche Diagnostics GmbH (Mannheim, Germany), and N,O-bis(trimethylsilyl)trifluoroacetamide was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). The reagents and microtiter plates used in the ELISA and analytical columns for HPLC and GC-MS have all been described previously (18–20).
Serum samples (0.1 mL) were treated with 0.6% aqueous glutamic acid solution and then applied to a solid-phase extraction column to separate the steroids into fractions of unconjugated and sulfoconjugated steroids. Next, both fractions were evaporated under vacuum until dry. The unconjugated steroids were further purified by HPLC based on the following retention times: cortisol (6.5 min), cortisone (6.8 min), 17-OH-pregnenolone (27.2 min), DHEA (30.8 min), 17-OH-progesterone (34.4 min), progesterone (56.7 min), and pregnenolone (63.0 min). The serum concentrations of pregnenolone, 17-OH-pregnenolone, and DHEA were measured by an ELISA and those of cortisol and cortisone were determined by GC-MS (18–20). The sulfoconjugated steroids were hydrolyzed with arylsulfatase and purified by HPLC. The isolated free steroids (17-OH-pregnenolone, DHEA, and pregnenolone) were derivatized with N,O-bis(trimethylsilyl)trifluoroacetamide and analyzed by GC-MS. The resulting values were expressed as the monosulfate concentration in serum. The intra- and interassay coefficients of variation in these assays were <6.6% and <9.8%, respectively.
Statistics and protocol.
All statistical analyses were performed using SPSS (Version 14.0J, SPSS Inc., Chicago, IL). Clinical indicators and hormone values did not show a normal distribution. Thus, a Mann-Whitney U test was applied for comparison of continuous clinical variables between groups. This nonparametric test was also used for comparison of hormone levels instead of using log-transformed data because the distribution of the total amount of precursors was not known. For categorical variables, Fisher's exact test was used. A significant difference was defined as p < 0.05.
The study was approved by the Ethics Committee of Tokyo Women's Medical University. All parents of preterm infants of gestational age less than 32 wks were fully informed about the study on admission, and the infants were selected as candidates for the study after written informed consent was obtained. Once the infants were entered into the study, the parent received a brief explanation of the clinical status of the infants after blood sampling.
RESULTS
Patient characteristics.
A total of 158 infants under 32 wk of gestational age were admitted to the NICU at Tokyo Women's Medical University between January 2004 and March 2006. Two were excluded from the study because of major congenital anomalies, nine died before discharge, and seven were excluded because of prior glucocorticoid treatment. Twelve infants were diagnosed with late-onset AOP and treated with hydrocortisone; one of these infants was subsequently excluded for lack of response to treatment and diagnosis of bacterial infection of the intestinal tract. Infants admitted during an additional 6-mo period were also considered eligible for the control group to permit recruitment of matched controls. During this 6-mo period, another 35 preterm infants were admitted to the NICU, and all except three infants who received glucocorticoids during the first week of life were considered eligible for inclusion as control infants. Eleven infants were selected as matched controls over the whole study period. No matched infant could be found for one patient with a gestational age of 22 wk and 3 d. This infant showed similar results to those of other patients in terms of steroid hormone profile, but ultimately was excluded from the study to balance the gestational age and background characteristics of the patient and control groups.
A total of 22 infants were analyzed in the study: 11 in the patient group and 11 in the control group. There were no significant differences between the characteristics of the two groups (Table 1), and the incidences of common neonatal morbidities (Table 2) also showed no differences between the groups. The patients recovered from all signs of adrenal insufficiency after a single dose of glucocorticoids.
Steroid hormone concentrations.
Steroid hormones were measured just before hydrocortisone administration in the patient group, and were compared with the concentrations measured in the same weeks after birth in the control group. The cortisol concentrations did not differ significantly between the groups: 6.6 ± 4.5 (7.8, 3.1–11.3, median and quartiles) μg/dL and 3.4 ± 2.7 (2.5, 1.4–5.5) μg/dL in the patient and control groups, respectively (Table 3). The relationship between cortisol and other hormones was also evaluated. The enzyme activity of 3β-hydroxysteroid dehydrogenase (3β-HSD) is impaired in preterm infants, and therefore, the amounts of precursor hormones that are substrates for 3β-HSD were compared between the groups (21). DHEA and DHEA sulfate are not direct substrates for the enzyme, but they are the main products from the precursors in the fetal adrenal gland. Post 3β-HSD steroid hormones (17-OH-progesterone and progesterone) were also examined (Table 3). Most precursor hormones in patients were at higher levels than expected, but there were variations between the infants. Therefore, the amount of total precursors was calculated as the sum of the concentrations of DHEA, 17-OH-pregnenolone, pregnenolone, and their sulfates for comparison between the groups. The total precursor concentrations were 72.2 ± 50.3 (60.1, 34.0–122.7) and 25.0 ± 28.5 (17.0, 1.8–30.6) μg/dL in the patient and control groups, respectively (Mann-Whitney U test, p < 0.017; Fig. 1).
Comparison of the concentrations of cortisol and total precursors to cortisol between the patient (n = 11) and control (n = 11) groups. Closed circles and lines represent the concentrations of cortisol and precursors to cortisol. Dehydroepiandrosterone, 17-OH-pregnenolone, pregnenolone, and their sulfates are included as precursors to cortisol via the enzyme 3β-hydroxysteroid dehydrogenase. Open circles with vertical bars represent means ± SD. p values were calculated by Mann-Whitney U test. *p = 0.017, **p = 0.082.
DISCUSSION
In this study, we found that serum cortisol concentrations did not differ between patients diagnosed with AOP after the first week of life and controls matched for gestational age. The cortisol concentrations were compatible with previous findings in infants beyond the first week of life (12,21–23), but contrary to the expectation that critically ill patients should have higher cortisol concentrations than normal individuals (24). However, we also found that infants diagnosed with AOP had significantly higher accumulation of cortisol precursors, suggesting limited 3β-HSD activity.
Fetal cortisol production is limited and insufficient to meet the need for rapid cellular proliferation (25), and premature delivery may result in a limited ability to produce cortisol (26,27). In this study, cortisol concentrations did not increase in preterm infants with AOP, but the infants responded to hydrocortisone administration with resolution of clinical signs of adrenal insufficiency. Thus, late-onset circulatory collapse caused by AOP is likely a result of relative adrenal insufficiency under excess stress; however, this phenomenon is transient and the preterm infants can cope with the increased stress upon development of full 3β-HSD activity in the adrenal gland.
The strengths of the study include the matching of infants with AOP to a group without AOP, and the multiple and simultaneous GC-MS analysis of serum steroid hormones in preterm infants. There have been several studies of the concentration of cortisol in preterm infants (8–13,21–23), but the cross-reactivity of antibodies used in immunoassays of cortisol makes it difficult to evaluate the cortisol concentration using this technique. The HPLC and GC-MS used in the current study allow multiple products to be measured simultaneously. Analysis of urinary steroid hormones by GC-MS has been reported (28), but for patients with AOP it is necessary to analyze serum samples and not urine specimens because of the acute changes that occur in AOP.
Impaired production of cortisol and accumulation of precursors for cortisol has been shown in preterm infants with CLD or those under stress (12). These data suggest that preterm infants with hypotension or CLD have a limited ability to synthesize cortisol under stress, and our data are consistent with this possibility. Preterm infants may be exposed to more stress than term infants, because of mechanical ventilation, oxygen treatment, or i.v. alimentation, and the adrenal gland in such infants may be fully stimulated. However, a low response of the hypothalamus-pituitary-adrenal system has also been reported in preterm infants under stress (29,30). Two infants in our study did not show an increase of cortisol precursors. These two infants might have demonstrated reduced production against stress, but we were unable to perform an adrenal stimulation test, which would have ruled out the possibility of a failed response of either the hypothalamus or pituitary in these infants.
The number of infants who develop AOP in the NICU is relatively high and seems to have increased in the last decades; however, the increased incidence might reflect changes in the accuracy of diagnosis. Even if infants after the first week of life have hypotension or other clinical signs such as hyponatremia, hyperkalemia, oliguria, and worsening of respiratory conditions, thus suggesting adrenal insufficiency, these manifestations may not reflect underlying glucocorticoid deprivation. Specifically, it is almost impossible to differentiate these conditions from the initial symptoms of septic shock, and sepsis, hypovolemia, or other conditions associated with circulatory instability must first be ruled out in infants with these symptoms (31).
Understanding the pathophysiology of adrenal insufficiency in preterm infants is important for improving treatment and preventing the disorder, and examination of adrenal function requires studies of hormonal conditions using simultaneous analysis of steroid hormones. Our results indicate that preterm infants develop late-onset AOP because of relative adrenal insufficiency under excess stress. However, this conclusion is preliminary because of the small patient population, and it is still unclear which preterm infants will develop AOP and when adrenal function will mature. Therefore, further evaluation of adrenal function in a larger number of preterm infants is required to examine these issues.
Abbreviations
- AOP:
-
adrenal insufficiency of prematurity
- CLD:
-
chronic lung disease
- DHEA:
-
dehydroepiandrosterone
- GC-MS:
-
gas chromatography-mass spectrometry
- 3β-HSD:
-
3β-hydroxysteroid dehydrogenase
- VLBW:
-
very low birth weight
References
Kusuda S, Fujimura M, Sakuma I, Aotani H, Kabe K, Itani Y, Ichiba H, Matsunami K, Nishida H Neonatal Research Network, Japan 2006 Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics 118: e1130–e1138.
Helbock HJ, Insoft RM, Conte FA 1993 Glucocorticoid-responsive hypotension in extremely low birth weight newborns. Pediatrics 92: 715–716
Fauser A, Pohlandt F, Bartmann P, Gortner L 1993 Rapid increase of blood pressure in extremely low birth weight infants after a single dose of dexamethasone. Eur J Pediatr 152: 354–356
Sasidharan P 1998 Role of corticosteroids in neonatal blood pressure homeostasis. Clin Perinatol 25: 723–740
Gaissmaier RE, Pohlandt F 1999 Single-dose dexamethasone treatment of hypotension in preterm infants. J Pediatr 134: 701–705
Ng PC, Lam CW, Fok TF, Lee CH, Ma KC, Chan IH 2001 Refractory hypotension in preterm infants with adrenocortical insufficiency. Arch Dis Child Fetal Neonatal Ed 84: F122–F124
Seri I, Tan R, Evans J 2001 Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 107: 1070–1074
Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA 1994 Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab 78: 266–270
Hanna CE, Jett PL, Laird MR, Mandel SH, LaFranchi SH, Reynolds JW 1997 Corticosteroid binding globulin, total serum cortisol, and stress in extremely low-birth-weight infants. Am J Perinatol 14: 201–204
Huysman MW, Hokken-Koelega AC, De Ridder MA, Sauer PJ 2000 Adrenal function in sick very preterm infants. Pediatr Res 48: 629–633
Heckmann M, Wudy SA, Haack D, Pohlandt F 2000 Serum cortisol concentrations in ill preterm infants less than 30 weeks gestational age. Acta Paediatr 89: 1098–1103
Watterberg KL, Gerdes JS, Cook KL 2001 Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res 50: 190–195
Fernandez E, Schrader R, Watterberg K 2005 Prevalence of low cortisol values in term and near-term infants with vasopressor-resistant hypotension. J Perinatol 25: 114–118
Ng PC, Lee CH, Lam CW, Ma KC, Fok TF, Chan IH, Wong E 2004 Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 89: F119–F126
Efird MM, Heerens AT, Gordon PV, Bose CL, Young DA 2005 A randomized-controlled trial of prophylactic hydrocortisone supplementation for the prevention of hypotension in extremely low birth weight infants. J Perinatol 25: 119–124
Ng PC, Lee CH, Bnur FL, Chan IH, Lee AW, Wong E, Chan HB, Lam CW, Lee BS, Fok TF 2006 A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 117: 367–375
Watterberg KL, Scott SM 1995 Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics 95: 120–125
Tagawa N, Nakata Y, Kusuda S, Kobayashi Y, Watanabe F 1997 Serum levels of 16-dehydropregnenolone sulfate during the early neonatal period. Biol Pharm Bull 20: 76–78
Tagawa N, Kusuda S, Kobayashi Y 1997 C16 hydroxylation of 3β-hydroxy-[Delta]5-steroids during the early neonatal period. Biol Pharm Bull 20: 1295–1299
Tagawa N, Kusuda S, Kobayashi Y 1999 16-Dehydropregnenolone 3-sulfate, its source and metabolism in the feto-placental unit. Biol Pharm Bull 22: 1262–1265
Wittekind CA, Arnold JD, Leslie GI, Luttrell B, Jones MP 1993 Longitudinal study of plasma ACTH and cortisol in very low birth weight infants in the first 8 weeks of life. Early Hum Dev 33: 191–200
Arnold J, Leslie G, Bowen J, Watters S, Kreutzmann D, Silink M 1997 Longitudinal study of plasma cortisol and 17-hydroxyprogesterone in very-low-birth-weight infants during the first 16 weeks of life. Biol Neonate 72: 148–155
Watterberg KL, Shaffer ML, Garland JS, Thilo EH, Mammel MC, Couser RJ, Aucott SW, Leach CL, Cole CH, Gerdes JS, Rozycki HJ, Backstrom C 2005 Effect of dose on response to adrenocorticotropin in extremely low birth weight infants. J Clin Endocrinol Metab 90: 6380–6385
Cooper MS, Stewart PM 2003 Corticosteroid insufficiency in acutely ill patients. N Engl J Med 348: 727–734
Mesiano S, Jaffe RB 1997 Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 18: 378–403
Pepe GJ, Albrecht ED 1990 Regulation of the primate fetal adrenal cortex. Endocr Rev 11: 151–176
Fujieda K, Faiman C, Feyes FI, Winter JS 1982 The control of steroidogenesis by human fetal adrenal cells in tissue culture. IV. The effect of exposure to placental steroids. J Clin Endocrinol Metab 54: 89–94
Heckmann M, Hartmann MF, Kampschulte B, Gack H, Bodeker RH, Gortner L, Wudy SA 2005 Cortisol production rates in preterm infants in relation to growth and illness: a noninvasive prospective study using gas chromatography-mass spectrometry. J Clin Endocrinol Metab 90: 5737–5742
Hanna CE, Keith LD, Colasurdo MA, Buffkin DC, Laird MR, Mandel SH, Cook DM, LaFranchi SH, Reynolds JW 1993 Hypothalamic pituitary adrenal function in the extremely low birth weight infant. J Clin Endocrinol Metab 76: 384–387
Brosnan PG 2001 The hypothalamic pituitary axis in the fetus and newborn. Semin Perinatol 25: 371–384
Seri I, Noori S 2005 Diagnosis and treatment of neonatal hypotension outside the transitional period. Early Hum Dev 81: 405–411
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported, in part, by a grant from the Ministry of Health, Labour and Welfare, Japan.
Rights and permissions
About this article
Cite this article
Masumoto, K., Kusuda, S., Aoyagi, H. et al. Comparison of Serum Cortisol Concentrations in Preterm Infants With or Without Late-Onset Circulatory Collapse due to Adrenal Insufficiency of Prematurity. Pediatr Res 63, 686–690 (2008). https://doi.org/10.1203/PDR.0b013e31816c8fcc
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e31816c8fcc
This article is cited by
-
Predicting iatrogenic adrenal insufficiency in neonates exposed to prolonged steroid courses: do cortisol levels help?
Journal of Perinatology (2024)
-
Presumed adrenal insufficiency in neonates treated with corticosteroids for the prevention of bronchopulmonary dysplasia
Journal of Perinatology (2022)
-
Late-onset Circulatory Collapse and Continuous Positive Airway Pressure are Useful Predictors of Treatment-requiring Retinopathy of Prematurity: A 9-year Retrospective Analysis
Scientific Reports (2017)
-
Trends in morbidity and mortality among very-low-birth-weight infants from 2003 to 2008 in Japan
Pediatric Research (2012)
-
Cardiovascular instability after patent ductus arteriosus ligation in preterm infants: the role of hydrocortisone
Journal of Perinatology (2012)