Abstract
Background
Systemic inflammation plays a key role in the development of bronchopulmonary dysplasia (BPD). Cortisol is known to dampen inflammation. However, adrenal function following preterm birth is characterized by insufficient cortisol levels for the degree of inflammation, and a relative abundancy of cortisol precursors. We investigated whether this pattern could contribute to the development of BPD in preterm infants born <30 weeks of gestation.
Methods
Cortisol, cortisone, 17-OH progesterone (17-OHP) and 11-deoxycortisol were measured in serum obtained at postnatal days 1, 3, 7, 14 and 28, using liquid-chromatography-tandem-mass-spectrometry. The presence of BPD was ascertained at 36 weeks postmenstrual age.
Results
Sixty-five infants were included for analysis, of whom 32 (49%) developed BPD. Preterm infants developing BPD, as compared to those without BPD, had higher levels of 17-OHP, 11-deoxycortisol and cortisone relative to cortisol in their first week of life, but not at birth or beyond day 7.
Conclusion
Preterm infants developing BPD had higher levels of cortisol precursors and cortisone relative to cortisol in their first week of life than infants without BPD. These findings suggest that BPD is preceded by an activated hypothalamus-pituitary-adrenal axis that could not meet the high cortisol demands, which may predispose to inflammation and BPD.
Impact
-
Relative adrenal insufficiency is common in the first weeks after preterm birth, resulting in insufficient cortisol production for the degree of inflammation and a relative abundance of cortisol precursors;
-
Whether this pattern contributes to the development of bronchopulmonary dysplasia (BPD) is not fully elucidated, since most studies focused on cortisol levels;
-
Preterm infants developing BPD had higher levels of cortisol precursors and cortisone relative to cortisol in the first week of life, suggestive of a hypothalamus-pituitary-adrenal-axis activation during BPD development which cannot meet the high cortisol demands in tissues;
-
This glucocorticoid pattern is likely to dispose to inflammation and BPD.
Similar content being viewed by others
Introduction
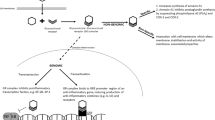
Bronchopulmonary dysplasia (BPD), a major complication of preterm birth, is associated with abnormal long term pulmonary and neurocognitive development, and systemic inflammation plays a central role in its pathophysiology.1 The adrenal cortex hormone cortisol is paramount for dampening inflammation, but among preterm infants in their first week of life relative adrenal insufficiency (RAI) is common, resulting in an insufficient production of cortisol relative to the degree of inflammation.2 One of the mechanisms behind RAI in preterm infants is immaturity and consequently a low activity of adrenal cortex enzymes, mainly 11β-hydroxylase.3 A low activity of adrenocortical enzymes not only results in a low cortisol secretory capacity, but also in a relative abundance of the cortisol precursor steroids 17-OH progesterone and 11-deoxycortisol. Evidence suggest that abundancy of these compounds may prevent cortisol from binding to the glucocorticoid receptor, by competitive receptor antagonism (Supplementary Fig. 1).4 Yet another mechanism contributing to RAI is a cortisol-cortisone shuttle that favors cortisone, i.e., the inactive form of cortisol, similar to fetal tissues.5 Whether this specific pattern predisposes to BPD, is not yet fully elucidated.6
Previous studies showed that preterm infants developing BPD, as compared to those who did not develop BPD, had lower serum cortisol levels between postnatal days 1 and 7,6,7 and a lower cortisol response to intravenous adrenocorticotropic hormone 1–24 (1.0 mcg/kg body weight) during the first 4 weeks of life.8,9 However, studies conducted to date only focused on the role of cortisol, among other steroid hormones, in the road to BPD, with the exception of one. Evidence from a randomized, placebo-controlled pilot study of low-dose hydrocortisone after preterm birth for the prevention of BPD showed that infants who eventually developed BPD had a higher 17-OH progesterone level at 12–48 h after birth (prior to the start of study medication) than infants without BPD, suggestive of adrenal cortex immaturity.10 Similarly, ill preterm infants, who are generally more likely to develop BPD, were found to have higher levels of cortisol precursors relative to cortisol than their healthy counterparts.11
Pharmacological treatment with postnatal systemic corticosteroids has shown to be effective for the prevention of BPD. However, postnatal administration of systemic dexamethasone, also increases the risk of neurodevelopmental impairment and cerebral palsy.12,13 Despite this controversy, postnatal corticosteroids remain a common treatment for preterm infants developing BPD, especially at younger gestational ages.14,15 Meta-regression analysis of placebo controlled randomized trials has shown that infants with a lower a priori risk of developing BPD are at risk of these long-term neurodevelopmental adverse effects.16 Therefore, corticosteroids should ideally only be administered to infants at high risk of developing BPD. However, identifying these high-risk infants is challenging, because current clinical prediction models for BPD lack quality accuracy.17,18 In our ongoing search for biomarkers predicting BPD, this prospective study followed preterm infants <30 weeks of gestation, with the aim to explore possible associations between adrenocortical output (based on a panel of cortisol, 17-OH progesterone, 11-deoxycortisol and cortisone measured in serum) throughout the first month of life and BPD ascertained at 36 weeks postmenstrual age (PMA).
Methods
Participants and setting
The PulmonaRy Inflammation and glucocorticoid sensitivity for the preDICTion of BronchoPulmonary Dysplasia (PRIDICT-BPD) study is a prospective cohort study, addressing the feasibility of various biomarkers for BPD. Infants were eligible for inclusion if they were born at less than 30 weeks gestation, and admitted to one of the two neonatal intensive care units (NICUs) of the Amsterdam University Medical Centers (Amsterdam UMC) between October 2019 and March 2021. Infants with congenital anomalies or who required major surgery within the first 24 h after birth were excluded. The Medical Research Ethics Committee of VU University Medical Center approved the study (protocol number 2019.371), and written informed consent was obtained from all parents.
Data collection and study procedures
For measurement of adrenal-cortex hormone levels, venous cord blood was obtained at birth, and capillary or arterial blood (depending on the presence of an indwelling arterial line) was obtained at postnatal days 3, 7, 14, and 28, in serum separator tubes, as long as the participants were admitted to the NICU. Blood draws were always combined with scheduled blood draws for standard care. After collection, the samples were centrifuged for 2 min at room temperature at 15,000 g, and serum aliquots were stored at −80 °C prior to analysis.
Data on maternal and neonatal characteristics were collected from obstetric and neonatal records (Table 1). BPD diagnosis was defined as the need for >21% oxygen for at least 28 cumulative days, and the severity of BPD was based on the need for oxygen or mode of respiratory support at 36 weeks postmenstrual age (PMA).19 Infants with mild, moderate or severe BPD were included in the BPD group, and their data were compared to the data of infants without BPD. Infants who died before the age of 36 weeks PMA, were excluded from the analysis.
Laboratory analysis
Serum concentrations of the adrenal-cortex hormones cortisol, cortisone, 17-OH progesterone and 11-deoxycortisol were measured at the Endocrine Laboratory of the Amsterdam UMC using an in-house Liquid Chromatography with tandem mass spectrometry (LC-MS-MS) method, as described earlier.20 Serum could be derived from either capillary or arterial blood. The lower limit of quatitation (LLOQ) was 0.5 nmol/L for cortisol, cortisone, 17-OH progesterone and 11-deoxycortisol. The intra-assay coefficient of variation was < 5% for all analytes. The inter-assay coefficient of variation was 3.9–4.5% for cortisol, 4.6–5.3% for cortisone, 7.4–7.5% for 17-OH progesterone and 6.0–6.3% for 11-deoxycortisol, at different levels.
Statistical analysis
Participant characteristics were compared between infants with and without BPD using a 2-sample t-test or a Mann-Whitney U test for continuous variables, and a Chi square-test or Fisher’s exact test for dichotomous variables, depending on the distribution of the data.
Outliers of adrenal-cortex hormone levels, caused by the administration of postnatal corticosteroids, were excluded from the analysis. Blood samples missing up to postnatal day 14 were considered missing at random, as this could always be attributed to logistic reasons. Missing data at postnatal day 28, when many infants were no longer admitted to the NICU, were not considered missing at random. Imputation of data missing at random was performed using multiple imputation with predictive mean matching, based on variables included in the imputation model and additional variables that showed substantial correlation (0.4–0.8) with the various hormone levels, including presence and duration of mechanical ventilation, and systolic and diastolic blood pressure within the first two weeks of life.
The distribution of hormonal data was skewed to the right, necessitating Ln transformation prior to analysis. Outcomes were compared using linear regression models. Next, based on previous studies showing correlation of GA, small for gestational age (SGA) and sex with both adrenal-cortex hormone levels and BPD, analyses were repeated after adjustment for these factors.6,7,21,22 SGA was defined as a birth weight below the 2.3th percentile according to the Hoftiezer curve.23 Linear regression analyses were performed on both the imputated data and the complete cases.
Statistical analyses were performed with SPSS Statistics (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp). The significance level was set at 0.05, and all tests were two-sided.
Results
Patient characteristics
During the inclusion period of the study, 150 preterm infants were found to be eligible, of whom 70 (52% males) were included (Fig. 1). Of these, two infants received hydrocortisone treatment at all times of sample collection, and three infants died before 36 weeks PMA and therefore a diagnosis of BPD could not be established in them, leaving 65 infants for analysis. Thirty-two (49%) of them developed BPD (44% mild, 9% moderate and 47% severe BPD). Infants with BPD were born at a lower GA, had a lower birthweight and were more often born SGA compared to infants without BPD. In addition, infants with BPD more often had morbidities, including treated persistent ductus arteriosus (PDA) and intraventricular hemorrhage (IVH) ≥ grade II, were more often treated with surfactant and dexamethasone, and spent more days on FiO2 > 0.21 and non-invasive respiratory support compared to infants without BPD (Table 1).
It was not always possible to obtain blood from participants at every time point, reflecting clinical practice, resulting in missing data of 33 (50.0%), 2 (3.1%), 11 (16.9%), 20 (30.8%) and 42 (65%) participants at postnatal days 1, 3, 7, 14 and 28, respectively. Furthermore, 5 infants were treated with postnatal corticosteroids (hydrocortisone or dexamethasone) at some point, and therefore their hormone levels from the start of this treatment were excluded for analysis (n = 7 samples (0 day 3 samples; 2 day 7 samples; 2 day 14 samples and 3 day 28 samples)).
Results of adrenocortical output between infants with and without BPD
Crude linear regression analyses showed similar cortisol levels at all time points between infants with and those without BPD. However, higher levels of cortisone at day 7, and of 17-OHP and 11-deoxycortisol at days 3 and 7 were found in infants with BPD compared to infants without BPD. In cord blood and at days 14 and 28 no differences in levels of cortisone, 17-OHP and 11-deoxycortisol were found (Fig. 2; Table 2).
After adjustment for GA, SGA and sex, we found significantly lower cortisol levels in infants with BPD compared to infants without BPD only at day 7. In addition, the differences in the levels of the other hormones found in crude analyses remained significant after adjustment, except for 17-OHP at day 3. Again, in cord blood and at days 14 and 28 no differences in adrenal cortex hormones were found (Fig. 2, Table 2). Complete-case analysis yielded similar results (Table 3, Supplementary material).
Discussion
In our study we showed that preterm infants developing BPD, as compared to preterm infants without BPD, had higher levels of cortisol precursors and cortisone relative to cortisol during the first week of life.
Although development of BPD follows a multifactorial pathophysiology, systematic inflammation plays a crucial role, and due to RAI many preterm infants are unable to raise their cortisol level in response to inflammation. Indeed, cortisol levels of ventilated preterm infants were inversely associated with pro-inflammatory cytokines in tracheal fluid, indicating that the link between RAI and BPD is likely to be explained by a disbalance in inflammation and anti-inflammation.6 However, despite this evidence, findings from studies examining associations between cortisol levels and BPD in preterm infants were controversial.24,25,26,27
The main message from our study is that RAI may not only be reflected by a low cortisol level. Among the mechanisms of RAI are immaturity of adrenal cortex enzymes, mainly 11β-hydroxylase, and a cortisol-cortisone shuttle that favors cortisone, in addition to prolonged suppression of hypothalamic corticotrophin-releasing hormone (CRH) following fetal exposure to excessive maternal glucocorticoids.3,28 Nevertheless, studies addressing associations between HPA axis activity and BPD in preterm infants almost exclusively measured cortisol of all adrenal cortex hormones, in spite of evidence from one study demonstrating that the 17-OHP level and the 17-OHP / 11-deoxycortisol ratio were higher 12–48 h after birth in those who eventually developed BPD.10 However, this study was limited to ventilated preterm infants. Our study using LC-MS/MS assay confirmed previous observations linking lower cortisol levels at day 7 to BPD in preterm infants, and added that multiple mechanisms may impede HPA axis activity during the first week of life in those infants who developed BPD. First, we found that infants with BPD had higher levels of the cortisol precursors 17-OH progesterone and 11-deoxycortisol in the first week of life. It has been demonstrated that cortisol precursors can bind to and activate the glucocorticoid receptor, but to a much lesser extent than cortisol.4,29 Through receptor competition, higher levels of cortisol precursors may prevent cortisol from binding to the glucocorticoid receptor, thereby attenuating its anti-inflammatory effect at tissue level.28 Second, we found that infants with BPD had higher levels of cortisone relative to cortisol in the first week of life, indicative of a cortisol-cortisone shuttle that favors cortisone.2,3,28 In cord blood and at postnatal days 14 and 28 no differences in adrenocortical hormone levels were found, which is compatible with the course of RAI, recovering before day 14.30
A strength of our study is that the adrenocortical hormones were measured with LC-MS-MS technology. Previous studies used competitive immuno-assays to measure steroid hormones, which carry a great risk of cross-reactivity with compounds that share the general structure with the hormone of interest.31 In conditions where cortisol precursors are elevated, for example in patients with congenital adrenal hyperplasia, cortisol levels as assessed by immunoassay were found to be falsely elevated due to cross reactivity.31,32,33 This phenomenon is not seen in LC-MS-MS analysis, which is therefore considered superior for the measurement of cortisol and other steroid hormones.32 Another strength of this study is that we corrected associations between adrenocortical hormone levels and BPD for GA, SGA and sex. These factors are known to cluster with both cortisol levels and BPD.6
A limitation of our study is the number of missing data, especially immediately after birth and at postnatal day 28. Missing data during the first 14 days were considered as missing at random, since these missing blood draws were missing mostly due to logistic reasons, reflecting daily practice. Therefore, we performed multiple imputation based on correlated variables. Although superior to whole-case analysis, to assure that the imputation did not influence the found associations, we also analyzed the data after including only those infants with complete data, which showed similar results. Missing data at day 28 were considered as missing not at random, being often attributed to earlier discharge from the NICU of healthier infants, and were therefore not imputed. Another limitation that needs to be considered is the relatively low number of infants included in this study. Given the explorative nature of our study, we did not perform a power analysis before start of the study. A post-hoc power analysis showed that the sample size of 70 infants is adequate with a power of 0.8 or higher to show significant differences in adrenal cortex hormone levels between groups.34,35,36,37
The results of this study raise the question as to whether adrenal cortex hormones can be included in a BPD prediction model. Since the beneficial effect of corticosteroid treatment to prevent BPD is dependent on the a-priori risk of developing BPD,16 early identification of high risk infants is urgently needed. However, systematic reviews on BPD prediction models showed that no model could accurately predict BPD.17 To our knowledge, only few BPD prediction models have included basal or peak cortisol levels as predictors,24,38 but none of them have included levels of cortisol precursors or cortisone, or used mass spectrometry to quantify levels of adrenal cortex hormones. Currently, we are conducting a multicenter study with a larger sample size, aimed at developing a prediction model of BPD based on, among others, adrenocortical hormone levels.
Conclusion
This study showed that preterm infants developing BPD had higher levels of cortisol precursors and cortisone relative to cortisol in the first week of life than preterm infants without BPD. These findings suggest that the development of BPD is accompanied by an activated hypothalamus-pituitary-adrenal axis that cannot meet the high cortisol demands in tissues. This biochemical profile is likely to predispose to inflammation and BPD.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ambalavanan, N. et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 123, 1132–1141 (2009).
Finken, M. J. J. et al. Programming of the hypothalamus-pituitary-adrenal axis by very preterm birth. Ann. Nutr. Metab. 70, 170–174 (2017).
Kamrath, C., Hartmann, M. F., Boettcher, C. & Wudy, S. A. Reduced Activity of 11beta-Hydroxylase Accounts for Elevated 17alpha-Hydroxyprogesterone in Preterms. J. Pediatr. 165, 280–284 (2014).
Pijnenburg-Kleizen, K. J. et al. Adrenal Steroid Metabolites Accumulating in Congenital Adrenal Hyperplasia Lead to Transactivation of the Glucocorticoid Receptor. Endocrinology 156, 3504–3510 (2015).
Stewart, P. M., Murry, B. A. & Mason, J. I. Type 2 11 Beta-Hydroxysteroid Dehydrogenase in Human Fetal Tissues. J. Clin. Endocrinol. Metab. 78, 1529–1532 (1994).
Watterberg, K. L., Scott, S. M., Backstrom, C., Gifford, K. L. & Cook, K. L. Links between Early Adrenal Function and Respiratory Outcome in Preterm Infants: Airway Inflammation and Patent Ductus Arteriosus. Pediatrics 105, 320–324 (2000).
Renolleau, C. et al. Association between Baseline Cortisol Serum Concentrations and the Effect of Prophylactic Hydrocortisone in Extremely Preterm Infants. J. Pediatr. 234, 65–70 e63 (2021).
Watterberg, K. L. & Scott, S. M. Evidence of early Adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics 95, 120–125 (1995).
Watterberg, K. L. et al. Effect of Dose on Response to Adrenocorticotropin in extremely low birth weight infants. J. Clin. Endocrinol. Metab. 90, 6380–6385 (2005).
Watterberg, K. L., Gerdes, J. S. & Cook, K. L. Impaired Glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr. Res 50, 190–195 (2001).
Finken, M. J. et al. Glucocorticoid programming in very preterm birth. Horm. Res. Paediatr. 85, 221–231 (2016).
Doyle, L. W., Cheong, J. L., Ehrenkranz, R. A. & Halliday, H. L. Early (<8 Days) Systemic Postnatal Corticosteroids for Prevention of Bronchopulmonary Dysplasia in Preterm Infants. Cochrane Database Syst. Rev. 10, CD001146 (2017).
Doyle, L. W., Cheong, J. L., Hay, S., Manley, B. J. & Halliday, H. L. Early (≪7 Days) Systemic Postnatal Corticosteroids for Prevention of Bronchopulmonary Dysplasia in Preterm Infants. Cochrane Database Syst. Rev. 10, CD001146 (2021).
Singh, N. & Gautham, K. S. Pattern of Postnatal Steroid Use for Bronchopulmonary Dysplasia in Extremely Preterm Infants. J. Perinatol. 42, 1258–1259 (2022).
Yao, S. et al. Postnatal Corticosteroid Use for Prevention or Treatment of Bronchopulmonary Dysplasia in England and Wales 2012-2019: A Retrospective Population Cohort Study. BMJ Open 12, e063835 (2022).
Doyle, L. W., Halliday, H. L., Ehrenkranz, R. A., Davis, P. G. & Sinclair, J. C. An Update on the Impact of Postnatal Systemic Corticosteroids on Mortality and Cerebral Palsy in Preterm Infants: Effect Modification by Risk of Bronchopulmonary Dysplasia. J. Pediatr. 165, 1258–1260 (2014).
Onland, W. et al. Clinical Prediction Models for Bronchopulmonary Dysplasia: A Systematic Review and External Validation Study. BMC Pediatr. 13, 207 (2013).
Romijn, M. et al. Prediction Models for Bronchopulmonary Dysplasia in Preterm Infants: A Systematic Review and Meta-Analysis. J. Pediatr. 113370 (2023). https://doi.org/10.1016/j.jpeds.2023.01.024. Epub ahead of print.
Jobe, A. H. The New Bronchopulmonary Dysplasia. Curr. Opin. Pediatr. 23, 167–172 (2011).
Fanelli, F. et al. Report from the Harmoster Study: Impact of Calibration on Comparability of Lc-Ms/Ms Measurement of Circulating Cortisol, 17oh-Progesterone and Aldosterone. Clin. Chem. Lab Med 60, 726–739 (2022).
Aoki, M., Urakami, T., Nagano, N., Aoki, R. & Morioka, I. Association of Plasma Cortisol Levels with Gestational Age and Anthropometric Values at Birth in Preterm Infants. Int J. Environ. Res Public Health 19, 11448 (2022).
Stoll, B. J. et al. Neonatal Outcomes of Extremely Preterm Infants from the Nichd Neonatal Research Network. Pediatrics 126, 443–456 (2010).
Hoftiezer, L. et al. From Population Reference to National Standard: New and Improved Birthweight Charts. Am. J. Obstet. Gynecol. 220, 383 e381–383 e317 (2019).
Banks, B. A. et al. Association of Plasma Cortisol and Chronic Lung Disease in Preterm Infants. Pediatrics 107, 494–498 (2001).
Nykanen, P., Anttila, E., Heinonen, K., Hallman, M. & Voutilainen, R. Early Hypoadrenalism in Premature Infants at Risk for Bronchopulmonary Dysplasia or Death. Acta Paediatr. 96, 1600–1605 (2007).
Pau, D. A., Mackley, A. & Bartoshesky, L. Newborn Screening Levels of 17-Hydroxyprogesterone in Very Low Birth Weight Infants and the Relationship to Chronic Lung Disease. J. Pediatr. Endocrinol. Metab. 19, 1119–1124 (2006).
Merz, U., Pfaffle, R., Peschgens, T. & Hornchen, H. The Hypothalamic-Pituitary-Adrenal Axis in Preterm Infants Weighing < or = 1250 G: Association with Perinatal Data and Chronic Lung Disease. Acta Paediatr. 87, 313–317 (1998).
Bolt, R. J. et al. Maturity of the Adrenal Cortex in Very Preterm Infants Is Related to Gestational Age. Pediatr. Res 52, 405–410 (2002).
Engels, M. et al. Glucocorticoid Activity of Adrenal Steroid Precursors in Untreated Patients with Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab 104, 5062–72 (2019).
Ng, P. C. et al. Transient Adrenocortical Insufficiency of Prematurity and Systemic Hypotension in Very Low Birthweight Infants. Arch. Dis. Child Fetal Neonatal Ed. 89, F119–F126 (2004).
Krasowski, M. D. et al. Cross-Reactivity of Steroid Hormone Immunoassays: Clinical Significance and Two-Dimensional Molecular Similarity Prediction. BMC Clin. Pathol. 14, 33 (2014).
Agrawal, N., Chakraborty, P. P., Sinha, A. & Maiti, A. False Elevation of Serum Cortisol in Chemiluminescence Immunoassay by Siemens Advia Centaur Xp System in 21-Hydroxylase Deficiency: An ‘Endocrine Laboma’. BMJ Case Rep. 13, e235450 (2020).
Haddad, R. A., Giacherio, D. & Barkan, A. L. Interpretation of Common Endocrine Laboratory Tests: Technical Pitfalls, Their Mechanisms and Practical Considerations. Clin. Diabetes Endocrinol. 5, 12 (2019).
Julious, S. A. Sample Sizes for Clinical Trials 317 (Chapman and Hall/CRC, 2010).
Chow, S,-C,, W. H., Shao, J,. Sample Size Calculations in Clinical Research 480 (Chapman and Hall/CRC, 2007).
Machin, D., C M. J., Tan, S. B., Tan, S. H. Sample Size Tables for Clinical Studies 2 edn(Wiley-Blackwell Science, 1997).
Zar, J. H. Biostatistical Analysis 2 edn(Practice-Hall, 1984).
Ng, P. C. et al. Early Pituitary-Adrenal Response and Respiratory Outcomes in Preterm Infants. Arch. Dis. Child Fetal Neonatal Ed. 89, F127–F130 (2004).
Funding
This study was supported by the Amsterdam Reproduction & Development Research Institute.
Author information
Authors and Affiliations
Contributions
M.R. made substantial contributions to conception and design, acquisition of data, data analysis and interpretation of data, drafted and revised the article, and approved the final version to be published. W.O. and M.J.J.F. made substantial contribution to conception and design, acquisition of data, data analysis and interpretation of data, revised the article critically, and approved the final version to be published. M.vanK. made substantial contribution to conception and design, acquisition of data, interpretation of data, revised the article critically and approved the final version to be published. A.C.H., J.R. and A.H.vanK. made substantial contributions to interpretation of the data, revised the manuscript critically for important intellectual content and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Written informed consent for participation and publication of the study results was obtained from all parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Romijn, M., Onland, W., van Keulen, B.J. et al. Glucocorticoid signature of preterm infants developing bronchopulmonary dysplasia. Pediatr Res 94, 1804–1809 (2023). https://doi.org/10.1038/s41390-023-02690-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02690-3