Abstract
Background
Most neonatal outcomes in neonates are related to normal adrenal gland function. Assessment of adrenal function in a sick preterm neonate remains a challenge, thus we hypothesized that adrenal steroid precursors to their product ratios have a direct relationship with neonatal outcomes.
Methods
We studied demographics of pregnancy and neonatal outcomes in 99 mother–infant pairs (24–41 weeks) and assayed 7 glucocorticoid precursors in the cortisol biosynthesis/degradation pathway. We correlated antenatal factors and short-term neonatal outcomes with these precursors and their ratios to assess maturity of individual enzymes.
Results
We found no correlation between cortisol levels with antenatal factors and outcomes. Antenatal steroid use impacted several cortisol precursors. 17-OH pregnenolone-to-cortisol ratio at birth was the best predictor of short-term neonatal outcomes, such as hypotension, RDS, IVH and PDA. A cord blood 17-OH pregnenolone:cortisol ratio of <0.21 predicts which neonate will have a normal outcome with a high sensitivity and specificity.
Conclusions
Maternal factors and antenatal steroids impact neonatal adrenal function and leads to maturation of adrenal function. 17-OH pregnenolone:cortisol ratio and not cortisol is the best predictor of adrenal function. Adrenal function can be assessed by evaluating the profile of adrenal steroids.
Similar content being viewed by others
Introduction
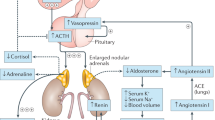
Adrenal glands play a significant role during gestation in fetal development, and postnatally, survival of the newborn depends on proper functioning of this endocrine gland to produce adequate amount of glucocorticoid hormones.1 Most preterm neonates have a state of relative adrenal insufficiency and assessment of adequacy of adrenal gland function in such infants remains a challenge in spite of major advances in neonatal care.2 Adrenal insufficiency due to congenital adrenal hyperplasia (CAH) or hypoplasia is diagnosed using established criteria by newborn screening programs3 and normative data on adrenal steroids and their precursors have been defined by several investigators.4 Several studies conducted over the past two decades have failed to come up with a reliable, trustworthy test for relative adrenal insufficiency in preterm neonates. This has resulted in arbitrary use or misuse of postnatal steroids, which may impact long-term neurodevelopmental outcomes of these infants.5 During gestation, the fetal adrenal grows exponentially till term and it consists of an outer “definitive zone” that produces mineralocorticoids, a thin ‘transitional zone” that secretes glucocorticoids at early gestational age (GA),6 and a much larger “fetal zone” that synthesizes androgenic precursors: dehydroepiandrosterone/sulfate and their 16-hydroxylated derivatives. These weak adrenal androgens are in turn converted to estriol by the placenta to support pregnancy.6,7 During maturation of adrenal gland, cells in the fetal zone undergo apoptosis, and after birth, fetal zone involutes and gets replaced by the transitional and definitive zones.8
Fetal zone expresses 3-β hydroxysteroid dehydrogenase type 2 (3-βHSD2) at very low levels limiting its ability to synthesize cortisol in significant amounts. Majority of steroid hormones produced during gestation are either weak androgens or relatively small amount of cortisol, which is rapidly inactivated by sulfation or locally in tissues by the action of 11-β hydroxysteroid dehydrogenase 1 (11-βHSD1) where it is converted to inactive cortisone.9 Maternal cortisol is inactivated by the actions of placental 11-βHSD2, thus helps protect the developing fetus from exposure to excessive amounts of maternal glucocorticoids during development.10,11 11-βHSD2 activity during later part of gestation is significant compared to 11-βHSD1 in human placenta and tissues, thus regulating the steroid milieu in fetal life.9,12 The fetal zone that normally regresses after birth may do so earlier in utero in response to several fetal–maternal factors, thus shape, size, and function of adrenal glands may change substantially during gestation in some infants.13 Thus, for the above-mentioned reasons, preterm neonates are born with an adrenal gland that is not capable of producing adequate amounts of cortisol to ensure extra-uterine survival.14,15 The large fetal zone of preterm neonate’s adrenal gland does not produce cortisol and the maturing transitional zones also has limited capacity to synthesize cortisol due to low expression of several enzymes, namely, 3-βHSD2, 17-hydroxylase, and 11-hydroxylase enzymes (Fig. 1).16 This leads to a buildup of several steroid precursors and a low level of cortisol, the final effector of glucocorticoid action. This situation in preterm neonates eventually improves postnatally but may take several weeks to months, during which these infants remain vulnerable to effects of relative glucocorticoid deficiency.17,18 This condition is also witnessed in some sick term infants who are infected or even in shock where the cortisol levels are low and cortisol precursor levels remain high due to inability to convert precursors to their final product cortisol.19,20
Simple schematic representation of relationship between maternal–placental–fetal compartments and steroid metabolism during gestation. 3β-HSD2 is the major rate-limiting enzyme that matures near term and allows buildup of precursors, which are subsequently converted to sulfated forms or undergo 16-hydroxylation; both processes lead to their inactivation
The pituitary gland of extremely low birth weight preterm infants like those born between 22 and 28 weeks have a limited capacity to secrete adrenocorticotropic hormone (ACTH) and using low-dose ACTH stimulation test where supra-physiological doses of ACTH are employed cannot help differentiate infants with relative glucocorticoid deficiency from normal.21,22 Thus we hypothesized that, to assess adrenal function in such infants, simultaneous measurement of cortisol and multiple other steroid precursors will provide more valuable information than a single assay and precursor-to-product ratio in glucocorticoid biosynthesis/degradation pathway may more accurately reflect maturity of individual enzymes. We hypothesized that immaturity of individual enzyme in preterm neonates in the glucocorticoid biosynthesis pathway will be reflected by buildup of precursor and a lower than normal product leading to higher precursor-to-product ratio. Thus ratio of precursor to product could be used as a clinically useful tool to assess maturity of the adrenal gland.
Methods
We conducted a prospective stratified observational study on 99 neonates born at a tertiary care center and admitted to our level IIIc neonatal intensive care unit or the newborn nursery at Vidant Medical Center, affiliated to East Carolina University, Greenville, NC. These infants were between 24 and 41 weeks’ gestation and infants were evenly spread over the range of GAs with 9–10 infants in each of the 2-week GA range grouped together (24–25, 26–27, 28–29, 30–31, 32–33, 34–35, 36–37, 38–39, 39–40, and >40 weeks) to obtain an even distribution of subjects. Once we had 9–10 subjects in each GA group, we stopped enrolling in that category till we had our estimated number of infants in each GA group. The study was approved by institutional review board of the East Carolina University, NC. Detailed maternal pregnancy and infant demographic data were collected at birth and neonatal morbidity and mortality data were collected till the time of discharge home. Infants with known chromosomal abnormalities, birth asphyxia (cord pH <7.0 and base excess >−15.0), known congenital adrenal hypoplasia or hyperplasia, and congenital heart disease were excluded. Infants were enrolled within 2–4 h after birth, and after obtaining parental consent, we processed their venous cord blood obtained at delivery. Cord blood was processed immediately by centrifugation for 15 min at 4 °C at 1500 × g to separate serum and stored at −20 °C until the time of transport to Endocrine Sciences laboratory (LabCorp), Calabasas Hills, CA in a single batch under dry ice using overnight express delivery. Cortisol, its precursors, and products were measured using liquid chromatography–tandem mass spectrometry (LC-MS/MS) as previously described.23 We measured a total of seven steroid precursors and their products in the cortisol biosynthesis/degradation pathway, namely progesterone, 17-hydroxypregnenolone, 17-hyrodxyprogesterone, 11-deoxycortisol, cortisol, corticosterone, and cortisone. We chose these seven steroid precursors since majority of these compounds are part of glucocorticoid biosynthesis pathway, and we also measured corticosterone, though not a glucocorticoid, as it has been shown to play a significant role during gestation and its levels affect fetal hypothalamic–pituitary–adrenal (HPA) axis and correlates with degree of fetal stress.24,25 Each infant was observed for development of hypotension, respiratory distress syndrome (RDS), intraventricular hemorrhage (IVH), and patent ductus arteriosus (PDA) based on standard clinical definitions. Various glucocorticoid precursors and cortisol ratios were calculated after converting their value into comparable units.
All data were stored in a secure RedCap® database during collection period and later exported to Excel and then SPSS (version 22.0) for analysis (IBM: Chicago, IL, USA). We correlated levels of each of these steroids and their ratios with fetal and maternal demographic data and neonatal outcomes. Descriptive statistics are depicted as median (interquartile range) and percentages, and when continuous variables were compared between two dichotomous variables, we used Mann–Whitney “U” test without adjustment for GA. Linear regressions were drawn for individual steroids and precursor-to-product ratio over GA range, where GA is the independent variable and steroid level like 17-hydroxyprogesterone level is the dependent variable. We have included all data points in our analysis and provided R2 and p value for the correlation. A receiver operating curve (ROC) analysis (with 95% confidence interval) was conducted to determine the cut-off ratio of 17-OH pregnenolone:cortisol that would best predict hypotension, RDS, IVH, and PDA. 17-OH pregnenolone:cortisol ratio was chosen since it reflects the activity of 3-βHSD2, 21-hydroxylase, and 11β-hydroxylase, the final three enzymes required to synthesize the end product cortisol (Fig. 1). Sensitivity and specificity analyses were conducted on different ratios to determine which ratio best predicts short-term neonatal outcomes.
Results
During the study period, 99 infant–mother pairs were studied, and their demographic and clinical details including prenatal and postnatal characteristics and short-term outcomes are summarized in Table 1. Subjects were evenly distributed across GA and birth weight range and reflect the overall population at our center. Serum cortisol levels in cord blood were similar between various group of infants (p = NS) except for slightly higher in female infants in comparison to male infants (p = 0.045) (Table 2). Antenatal steroids had a significant impact on cortisol precursors and was associated with lower levels of 17-OHP, the commonly measured precursor by most newborn screening programs for CAH (Figs. 2 and 3a). Lower level of 17-OHP was noted even though these infants were of lower GA and birth weights in comparison to infants closer to term GA and higher birth weights since this population of infants were exposed to antenatal steroids (p < 0.001). When the ratio of 17-OH pregnenolone:cortisol was examined, the ratio was much higher at lower GA and birth weight range compared to higher GA and birth weight category of infants who did not receive antenatal steroids (p < 0.001) (Fig. 3b). A similar trend was observed for other precursors and precursor-to-cortisol ratios (data not shown). We did not find any significant correlation between demographic factors or cortisol to its inactive metabolites cortisone or the fetal stress hormone corticosterone unlike other published reports.24
Effect of antenatal betamethasone on steroid precursors and cortisol values and their ratio in cord blood. a 17-OH progesterone cord blood levels were significantly lower in infants who received antenatal steroids (p < 0.001). b 17-OH pregnenolone:cortisol ratio were significantly higher in infants who received antenatal steroids (p < 0.0001)
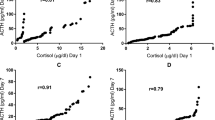
We found a significant direct relationship between several steroid precursors, their ratio to cortisol, and short-term neonatal outcomes, such as hypotension, RDS, IVH, and PDA (Table 3). Cord blood cortisol levels had no correlation to these outcomes (p > 0.10). The ratios of 17-hydroxypregnenolone to cortisol and of 17 hydroxyprogesterone to cortisol both were significantly predictive of short-term outcomes, such as hypotension, RDS, IVH, and PDA (p ≤ 0.05). With 60 subjects in the RDS group and 38 non-RDS patients, Mann–Whitney U test with a significance level of 0.05 achieves >99% power to detect the effects shown in our analysis and similar power was achieved for other outcomes, such as IVH and PDA. An ROC drawn between 17-hydroxypregnenolone-to-cortisol ratio and outcomes such as RDS and PDA showed that a ratio of <0.21 can predict RDS with a sensitivity of 60% and specificity of 79%, with an area under the curve of 0.68 (p = 0.01) (Fig. 4a). Likewise, 17-hydroxypregnenolone-to-cortisol ratio of <0.21 can predict PDA with a sensitivity of 83% and specificity of 65%, with an area under the curve of 0.80 (p = 0.001) (Fig. 4b). Similar ROC curves were drawn with 17-OH progesterone-to-cortisol ratio with lower sensitivity and specificity (data not shown).
Discussion
Our study has for the first time presented evidence to show that profiling precursors in the cortisol biosynthesis pathway could be helpful in predicting short-term neonatal outcomes and that cord blood levels of cortisol is not a sensitive measure of adequacy of adrenal function. Our study is in agreement with other reports suggesting that blood levels of cortisol lacks the ability to predict short-term outcomes.26,27 17-Hydroxyprogestrone levels obtained as part of day 2–3 newborn screening program is elevated in several preterm neonates, which ultimately normalizes on repeat testing around 4–5 months of age.28 Other investigators have utilized steroid profiling and precursor:product ratios using LC-MS to help differentiate false positive cases of CAH from the true positives.29,30,31 In our report, we have focused on the utility of cortisol precursor-to-cortisol ratio as a measure of adrenal function, which may be a guide for selecting patients who will benefit the most from postnatal glucocorticoid supplementation. Profiling steroids can also be utilized to assess maturity of individual enzymes in the biosynthesis pathway, where a lower precursor-to-product ratio indicates a more mature or efficiently functioning enzyme. This technique could be used to provide a rational basis for clinical supplementation of hydrocortisone in preterm and sick term neonates.
Our study has demonstrated that a random cortisol level does not predict any short-term outcomes or help manage sick neonates unless it is very high when it rules out inadequacy of adrenal glucocorticoids as the cause for the clinical problem. The classic low-dose ACTH stimulation tests used clinically use supraphysiologic doses of ACTH to stimulate adrenal glands to produce cortisol, so its utility is limited to diagnosing mainly cases of complete adrenal absence or failure.32 These ACTH stimulation tests fail to differentiate preterm and sick term newborns who are unable to mount an adequate adrenal response in cases of severe illness or sepsis33,34 compared to those who lack adrenal function altogether.
Our study sheds light on maturity of fetal adrenal gland, which is mainly attributed to their immaturity and immaturity of their HPA axis could also play a role and both conditions may be equally responsible for the observed clinical picture. Our data have shown that preterm neonates had lower levels of steroid precursors if they received antenatal betamethasone treatment compared to others who did not. This is in contrast with reports from pre-antenatal steroid era where extremely preterm neonates had very high levels of cortisol precursors compared to mature term neonates on their newborn screening test.35,36 Antenatal betamethasone and maternal stress both lead to changes in adrenal steroid profile, which directly affects neonatal outcomes.37,38,39 Maturation and control of gene expression of enzymes involved in cortisol biosynthesis, namely, 3βHSD2, 21-hydroxylase, and 11-β hydroxylase, occurs via ACTH through c-AMP-mediated response element in their promoters, while several other growth factors like insulin-like growth factors also play a significant role.10,40 3βHSD2 gene expression is controlled by glucocorticoids via their action through stat5 activation.40 3βHSD2 is the major rate-limiting enzyme in cortisol biosynthesis and exogenous glucocorticoids acts on its promoter via stat5 to upregulate its expression, which explains lower precursor buildup seen in our cases who received antenatal betamethasone. This phenomenon apparently will occur during postnatal steroid use as well, leading to maturation of adrenal gland function. We have shown that 17-hydroxypregnenolone-to-cortisol ratio can predict which infants will avoid having complications such as hypotension, RDS, IVH, and PDA. Measurement of this ratio can help clinicians to choose infants who will truly benefit from postnatal hydrocortisone supplementation.
Our study has certain limitations. It is a single-center study with small number of subjects and our assessment of adrenal function is limited to the time around birth due to the use of cord blood samples only. Thus we were able to predict only short-term outcomes by using our steroid profile data and it was not possible to predict long-term outcomes where there is a need to repeat this test on multiple occasions postnatally, especially those neonates who undergo stressful events like surgery or who develop sepsis. Our study will serve as preliminary data to help plan more elaborate studies to test this hypothesis further. In conclusion, tandem mass spectrometry (LC-MS/MS) can provide profile of several steroids from a single blood sample and this information can better define maturity of adrenal gland and help identify infants who need replacement glucocorticoid therapy to improve outcomes.
References
Watterberg, K. L. Adrenocortical function and dysfunction in the fetus and neonate. Semin. Neonatol. 9, 13–21 (2004).
Fernandez, E. F. & Watterberg, K. L. Relative adrenal insufficiency in the preterm and term infant. J. Perinatol. 29 (Suppl 2), S44–S49 (2009).
Speiser, P. W. et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 103, 4043–4088 (2018).
Greaves, R. F. et al. Establishment of hormone reference intervals for infants born <30weeks’ gestation. Clin. Biochem. 47, 101–108 (2014).
Jobe, A. H. Glucocorticoids in perinatal medicine: misguided rockets? J. Pediatr. 137, 1–3 (2000).
Ishimoto, H. & Jaffe, R. B. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr. Rev. 32, 317–355 (2011).
Hammer, G. D., Parker, K. L. & Schimmer, B. P. Minireview: Transcriptional regulation of adrenocortical development. Endocrinology 146, 1018–1024 (2005).
Watterberg, K. & Muglia, L. J. Fetal and Neonatal Physiology (Elsevier, Philadelphia, PA, 2017).
Miller, W. L. & Auchus, R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Tomlinson, J. W. et al. 11β-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 25, 831–866 (2004).
Benediktsson, R., Calder, A. A., Edwards, C. R. & Seckl, J. R. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 46, 161–166 (1997).
Green, B. B. et al. The role of placental 11-beta hydroxysteroid dehydrogenase type 1 and type 2 methylation on gene expression and infant birth weight. Biol. Reprod. 92, 149 (2015).
Karsli, T. et al. Assessment of neonatal adrenal size using high resolution 2D ultrasound and its correlation with birth demographics and clinical outcomes. J. Matern. Fetal Neonatal Med. 32, 377–383 (2017).
El-Khuffash, A., McNamara, P. J., Lapointe, A. & Jain, A. Adrenal function in preterm infants undergoing patent ductus arteriosus ligation. Neonatology 104, 28–33 (2013).
Huysman, M. W. A., Hokken-Koelega, A. C. S., Ridder, M. A. J. & Sauer, P. J. J. Adrenal function in sick very preterm infants. Pediatr. Res. 48, 629–633 (2000).
Coulter, C. L. et al. Functional maturation of the primate fetal adrenal in vivo. II. Ontogeny of corticosteroid synthesis is dependent upon specific zonal expression of 3 beta-hydroxysteroid dehydrogenase/isomerase. Endocrinology 137, 4953–4959 (1996).
Watterberg, K. L., Gerdes, J. S. & Cook, K. L. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr. Res. 50, 190–195 (2001).
Aucott, S. W., Watterberg, K. L., Shaffer, M. L., Donohue, P. K. & PROPHET study group. Early cortisol values and long-term outcomes in extremely low birth weight infants. J. Perinatol. 30, 484 (2009).
Khashana, A., Ojaniemi, M., Leskinen, M., Saarela, T. & Hallman, M. Term neonates with infection and shock display high cortisol precursors despite low levels of normal cortisol. Acta Paediatr. 105, 154–158 (2016).
Khashana, A. et al. Cortisol precursors in neonates with vasopressor-resistant hypotension in relationship to demographic characteristics. J. Matern. Fetal Neonatal Med. 31, 2473–2477 (2018).
Soliman, A. T. et al. Circulating adrenocorticotropic hormone (ACTH) and cortisol concentrations in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: cortisol response to low-dose and standard-dose ACTH tests. Metabolism 53, 209–214 (2004).
Ng, P. C. et al. Transient adrenocortical insufficiency of prematurity and systemic hypotension in very low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 89, F119–F126 (2004).
Taylor, A. E., Keevil, B. & Huhtaniemi, I. T. Mass spectrometry and immunoassay: how to measure steroid hormones today and tomorrow. Eur. J. Endocrinol. 173, D1–D12 (2015).
Wynne-Edwards, K. E., Edwards, H. E. & Hancock, T. M. The human fetus preferentially secretes corticosterone, rather than cortisol, in response to intra-partum stressors. PLoS ONE 8, e63684 (2013).
Raubenheimer, P. J., Young, E. A., Andrew, R. & Seckl, J. R. The role of corticosterone in human hypothalamic-pituitary-adrenal axis feedback. Clin. Endocrinol. (Oxf.) 65, 22–26 (2006).
Aucott, S. W., Watterberg, K. L., Shaffer, M. L., Donohue, P. K. & Group, P. Do cortisol concentrations predict short-term outcomes in extremely low birth weight infants? Pediatrics 122, 775–781 (2008).
Khashana, A., Saarela, T., Ramet, M. & Hallman, M. Cortisol intermediates and hydrocortisone responsiveness in critical neonatal disease. J. Matern. Fetal Neonatal Med. 30, 1721–1725 (2017).
Anandi, V. S. & Shaila, B. Evaluation of factors associated with elevated newborn 17-hydroxyprogesterone levels. J. Pediatr. Endocrinol. Metab. 30, 677–681. (2017).
Tieh, P. Y., Yee, J. K., Hicks, R. A., Mao, C. S. & Lee, W. N. Utility of a precursor-to-product ratio in the evaluation of presumptive positives in newborn screening of congenital adrenal hyperplasia. J. Perinatol. 37, 283–287 (2017).
Seo, J. et al. Steroid profiling for congenital adrenal hyperplasia by tandem mass spectrometry as a second-tier test reduces follow-up burdens in a tertiary care hospital: a retrospective and prospective evaluation. J. Perinat. Med. 42, 121–127 (2013).
Hicks, R. A. et al. Precursor-to-product ratios reflect biochemical phenotype in congenital adrenal hyperplasia. Metabolomics 10, 123–131 (2014).
Karsli, T., Sutter, J. & Shekhawat, P. S. X-linked adrenal hypoplasia congenita due to NR0B1 (DAX1) deficiency presenting as severe respiratory distress in near term infants. Pediatr. Neonatol. 57, 444–445 (2016).
Fernandez, E. F., Montman, R. & Watterberg, K. L. ACTH and cortisol response to critical illness in term and late preterm newborns. J. Perinatol. 28, 797–802 (2008).
Hochwald, O., Holsti, L. & Osiovich, H. The use of an early ACTH test to identify hypoadrenalism-related hypotension in low birth weight infants. J. Perinatol. 32, 412–417 (2012).
Kwon, C. & Farrell, P. M. The magnitude and challenge of false-positive newborn screening test results. Arch. Pediatr. Adolesc. Med. 154, 714–718 (2000).
al Saedi, S., Dean, H., Dent, W. & Cronin, C. Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. J. Pediatr. 126, 985–987 (1995).
Rauh, M. Steroid measurement with LC-MS/MS. Application examples in pediatrics. J. Steroid Biochem. Mol. Biol. 121, 520–527 (2010).
Millage, A. R., Latuga, M. S. & Aschner, J. L. Effect of perinatal glucocorticoids on vascular health and disease. Pediatr. Res. 81, 4–10 (2017).
Buyukkayhan, D., Ozturk, M. A., Kurtoglu, S., Koklu, E. & Yikilmaz, A. Effect of antenatal betamethasone use on adrenal gland size and endogenous cortisol and 17-hydroxyprogesterone in preterm neonates. J. Pediatr. Endocrinol. Metab. 22, 1027–1031 (2009).
Simard, J. et al. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr. Rev. 26, 525–582 (2005).
Acknowledgements
We would like to express our gratitude to Dr. Samuel H Pepkowitz and Dr. Donald W Chandler from Endocrine Sciences Laboratory (LabCorp.) for their contribution in assaying our samples using the latest mass spectrometry technique in a blinded fashion. This study was internally funded by the Department of Pediatrics & Obstetrics & Gynecology, East Carolina University, Greenville, NC and steroid assays were performed by Endocrine Sciences (LabCorp) Laboratories.
Author information
Authors and Affiliations
Contributions
T.K. was responsible for conducting the study, data collection, data analysis, and writing initial draft of manuscript. V.G.J., Q.W., and M.M. were responsible for data analysis, preparing tables and figures, and helped write the initial draft of the manuscript. S.H.P. and D.W.C. did sample processing and assayed steroid hormones. P.S.S. was responsible for study design, data analysis, data interpretation, and wrote the final version of the manuscript. All authors approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karsli, T., Jain, V.G., Mhanna, M. et al. Assessment of adrenal function at birth using adrenal glucocorticoid precursor to product ratios to predict short-term neonatal outcomes. Pediatr Res 87, 767–772 (2020). https://doi.org/10.1038/s41390-019-0629-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0629-8