Abstract
Near-infrared spectroscopy has been used for measurement of changes in cerebral Hb concentrations in infants to study cerebral oxygenation and hemodynamics. In this study, measurements by time-resolved spectroscopy (TRS) were performed in 22 neonates to estimate the values of light absorption coefficient and reduced scattering coefficient (μ's), cerebral Hb oxygen saturation (Sco2), cerebral blood volume (CBV), and differential pathlength factor (DPF), and the relationships between postconceptional age and μ's, Sco2, CBV, and DPF were investigated. A portable three-wavelength TRS system with a probe attached to the head of the neonate was used. The mean μ's values at 761, 795, and 835 nm in neonates were estimated to be (mean ± SD) 6.46 ± 1.21, 5.90 ± 1.15 and 6.40 ± 1.16/cm, respectively. There was a significant positive relationship between postconceptional age and μ's at those three wavelengths. The mean Sco2 value was calculated to be 70.0 ± 4.6%, and postconceptional age and Sco2 showed a negative linear relationship. The mean value of CBV was 2.31 ± 0.56 mL/100 g. There was a significant positive relationship between postconceptional age and CBV. The mean DPF values at 761, 795, and 835 nm were estimated to be 4.58 ± 0.41, 4.64 ± 0.46, and 4.31 ± 0.42, respectively. There was no relationship between postconceptional age and DPF at those three wavelengths. The results demonstrated that our near-infrared TRS method can be used to monitor μ's, Sco2, CBV, and DPF in the neonatal brain at the bedside in an intensive care unit.
Similar content being viewed by others
Main
During the perinatal period, the brain undergoes anatomic, functional, and metabolic changes. The anatomic changes include neuronal proliferation, migration, organization, and myelination, and the metabolic changes match the process of initial overproduction and subsequent elimination of excessive neurons, synapses, and dendritic spines known to occur in the developing brain. Noninvasive assessment of cerebral anatomic changes and of oxygen delivery and utilization is useful for evaluating the effectiveness of therapy and for preventing oxygen toxicity in seriously ill neonates.
Near-infrared spectroscopy (NIRS) has been used in the clinical field with various measuring devices using several wavelengths. A method using continuous-wave NIRS has been developed and reported to be suitable for clinical use in infants (1–7). However, current commercially available NIRS systems can detect only changes in cerebral Hb. Because NIRS is based on the modified Beer-Lambert law, a change in hematocrit and blood volume as well as developmental and pathophysiologic changes in brain tissue affect the pathlength of near-infrared light. In a few recent studies, absolute values of cerebral Hb oxygen saturation (Sco2) and cerebral blood volume (CBV) in infants were measured without inducing Hb concentration changes by using full-spectral near-infrared spectroscopy (8–11) and spatially resolved spectroscopy (12). However, these devices can measure only light absorption coefficient (μa), which represents the physiologic state, particularly the Hb concentration and oxygen saturation.
A recently developed time-resolved spectroscopy (TRS) device enables simultaneously quantitative analysis of μa and light-reduced scattering coefficient (μ's) in tissue by using the photon diffusion theory. μ's is thought to be a new parameter for assessment of structural changes in the brain, such as brain edema and myelination. Although TRS has been used in neonates, there have only been a few reports on its use in neonates, and measurements in neonates at the bedside have not been possible because of the size and the cost of typical laboratory equipment needed for these measurements. However, a new TRS device that is portable and has a high data acquisition rate was used clinically recently. This TRS system can be used 1) for continuous absolute quantification of hemodynamic variables and 2) for better estimation of light-scattering properties by measurement of μ's and differential pathlength factor (DPF). The aim of this study was to measure the values of μ's, μa, Sco2, CBV, and DPF in neonates using TRS and to determine the relationships between postconceptional age and μ's, Sco2, CBV, and DPF.
METHODS
Patient population.
Measurements were carried out in 27 neonates who were undergoing neonatal intensive care at the Maternal and Perinatal Center of Kagawa University Hospital. Written informed consent was obtained from the parents of each neonate. The study was also approved by a local ethics committee. Data from five neonates were excluded from the analysis because the measurements were affected by movement artifacts. Successful measurements were performed in 22 neonates. Their gestational age (mean ± SD) was 36.8 ± 3.1 wk, and birth weight was 2365 ± 791 g. The mean time after birth when measurements were carried out was 32.9 ± 21.1 h. The clinical diagnosis of each neonate is shown in Table 1. Mechanical ventilation was required in four neonates, and three neonates received catecholamines during this study. No neonates have problems in their prognosis at present.
Near-infrared time-resolved spectroscopy system and analysis.
We used a portable three-wavelength TRS system (TRS-10; Hamamatsu Photonics K.K., Hamamatsu, Japan) and attached a probe to the forehead of each neonate. In the TRS system, a time-correlated single-photon-counting technique is used for detection. The system is controlled by a computer through a digital I/O interface that consists of a three-wavelength (761, 795, and 835 nm) picosecond light pulser (PLP) as the pulse light source, a photon-counting head for single photon detection, and signal-processing circuits for time-resolved measurement. The PLP emits NIR light with a pulse duration of ∼100 ps and an average power of at least 150 μW at each wavelength at repetition of 5 MHz. The input light power to the patient was ∼20 μW.
The light from the PLP is sent to a patient by a source fiber with a length of 3 m, and the photon re-emitted from the patient is collected simultaneously by a detector fiber bundle with a length of 3 m (13). The light source fiber used in this study was a graded-index-type single fiber with a numerical aperture of 0.25 and a core diameter of 200 μm, and the light detector fiber was a bundle fiber with a diameter of 3 mm and numerical aperture of 0.21. Finally, a set of histograms of photon flight time, which is called a re-emission profile, is recorded (14,15). One temporal re-emission profile includes 1024 time channels that span ∼10 ns with a time step of ∼10 ps. In this study, the emerging light was collected over a period of 1 s to exceed at least 1000 count of photon at the peak channel of the re-emission profiles. The instrumental response was measured with the input fiber placed opposite the receiving fiber through a neutral density filter. The instrumental response of the TRS system was ∼150 ps Full-Width Half-Maximum at each wavelength.
The re-emission profiles observed at each measurement point were fitted by the photon diffusion equation proposed by Patterson et al. (16) to calculate the values of μa and μ's of the head at wavelengths of 761, 795, and 835 nm. In the fitting procedure, a nonlinear least squares fitting method based on Levenberg-Marquardt's method was used. In each iterative calculation, the function from the photon diffusion equation in reflectance mode, which was convoluted with the instrumental response, was fitted to the observed re-emission profile. The calculation regions were determined to include the observed profile data, and data of 600 channels were included into the fit.
After determination of the values of μa at three wavelengths, the oxyHb and deoxyHb concentrations were calculated from the absorption coefficients of oxyHb and deoxyHb using the following equations with the assumption that background absorption is due only to 85% (by volume) water (8):
MATH

In these equations, is the extinction coefficient at λ nm, and [oxyHb] and [deoxyHb] are concentration of oxyHb and deoxyHb, respectively.
First, water absorption was subtracted from μa at each of the three wavelengths, and then the concentrations of oxyHb and deoxyHb were estimated using the least squares fitting method. The absorption coefficients for oxyHb, deoxyHb, and water shown in Table 2 were used.
Cerebral total Hb (totalHb) concentration, Sco2, and CBV were calculated as follows:
MATH

where [ ] indicates Hb concentration (μM), MWHb is the molecular weight of Hb (64,500), tHb is venous Hb concentration (g/dL), and Dt is brain tissue density (1.05 g/mL).
The mean pathlength was calculated from the difference between the center of gravity of the measured reemission profile and that of the instrumental function. We assumed that the value for the refractive index of brain tissue is 1.4 (17). The ratio of optical pathlength to interoptode distance is defined as the DPF (18).
All of the neonates were in the supine position during measurements. Their condition was stable, and they were sleeping during the procedure at least 12 min. The optode positions were on the forehead of each neonate, and interoptode space was 26–32 mm. At the same time, oxygen saturation by pulse oximeter (Spo2) was monitored using a Nellcor N550 (Tyco, Tokyo, Japan).
The average values of μ's, Sco2, CBV, and DPF for each patient were calculated for a period of 5 min during the 12-min measurement period in a steady state not affected by movement artifacts. Variation, particularly in cerebral oxygen delivery, can occur over short periods, and long averaging measurement time is needed. Sco2 depends on cerebral oxygen delivery and extraction, both of which vary with postconceptional age and postnatal age. We previously reported postnatal changes in CBV and Sco2 in normal infants determined by full-spectrum NIRS. CBV and Sco2 changed within the first 15 min after birth (10,11), but remained constant from 12 h after birth until day 5 (9). In the present study, the average time at which measurements were carried out after birth was 33 ± 21 h, and the values of Sco2 and CBV in this study therefore were not affected by birth stress. In a previous study, the cerebral blood velocity in infants measured by Doppler ultrasound showed cyclical fluctuations with frequency ranging from 1.5 to 5 cycles/min (19). NIRS studies have also shown oscillations of the Hb oxygenation state with frequency ranging from 3 to 5 cycles/min (20). Therefore, the use of average values for a period of 5 min in each neonate in this study seems sufficient for estimation of cerebral Hb in a steady state.
Statistical analysis.
A StatView-J 5.0 package for the Macintosh computer was used for statistical analysis. The level of statistical significance was set at a probability of p < 0.05 for all tests. All measurement results are expressed as means ± SDs.
RESULTS
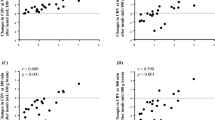
The values of DPF, μa, μ's, Sco2, totalHb, and CBV are shown in Table 3. The values of μ's in the 22 neonates at 761, 795, and 835 nm were estimated to be (mean ± SD) 6.46 ± 1.21, 5.90 ± 1.15, and 6.40 ± 1.16/cm, respectively. As shown in Fig. 1, there was a significant positive relationship between postconceptional age and μ's at the three wavelengths.
Relationships between postconceptional age and μ's at 761, 795, and 835 nm in 22 neonates. The relationships between postconceptional age and μ's at 761, 795, and 835 nm were y 761 nm = 0.293x − 4.40 (r = 0.742, p < 0.001), y 795 nm = 0.202x − 1.58 (r = 0.539, p = 0.010), and y 835 nm = 0.298x − 4.65 (r = 0.787, p < 0.001), respectively.
Mean Sco2 was 70.0 ± 4.6% (range 60.8–78.8%), and mean Spo2 was 98.6% (range 92–100%). Postconceptional age and Sco2 showed a significant negative linear relationship as shown in Fig. 2.
There was a significant positive relationship between postconceptional age and cerebral totalHb. The relationship between postconceptional age and cerebral totalHb was y = 4.23x + 91.9 (r = 686, p < 0.001). The mean CBV was 2.31 ± 0.56 mL/100 g (range 1.42–3.40 mL/100 g). As shown in Fig. 3, there was a significant positive relationship between postconceptional age and CBV. There was no relationship between blood Hb concentration and CBV.
The DPF values at 761, 795, and 835 nm were estimated to be 4.58 ± 0.41, 4.64 ± 0.46, and 4.31 ± 0.42, respectively. There was no relationship between postconceptional age and DPF at any of the three wavelengths.
DISCUSSION
This is the first report on the relationship between postconceptional age and μ's in the neonatal brain. We previously reported that there were no significant differences between the values of μ's at each wavelength for inspired fractional O2 levels in the range of 4–100% in a piglet hypoxia model. These results are similar to those obtained in a study by Zhang et al. (21) showing that scattering changes detected by frequency-domain spectroscopy were associated only with asphyxia and death. Yamashita et al. (22) reported the results of a preliminary study on μ's in the piglet brain by using TRS. Their results showed a notable decrease after death. Tissue edema and structural changes occur during severe hypoxia, particularly at and after death, and values of μ's are thought to change only during structural changes in tissue as a result of cerebral energy failure. Furthermore, developmental changes in the brain, especially neuronal proliferation, migration, organization, and myelination, were thought to be related to the positive relationship between μ's and postconceptional age. However, at present, magnetic resonance imaging techniques enable a much better assessment of anatomic development in infants.
Values of Sco2 in infants that were obtained in previous studies using NIRS (8–12,23,24) are summarized in Table 4. Mean Sco2 in the 22 neonates in this study was 70.0 ± 4.6%, and the range of Sco2 values was small (60.8–78.8%). This range is similar to those previously reported (63–69%) in infants (8–12,23). Results of measurements using the TRS system also showed a decrease in Sco2 with increasing postconceptional age. Sco2 measured by NIRS represents a mixed vascular Hb oxygen saturation of capillaries, arteriae, and veins in that tissue field. Absolute measurements are made on the basis of the assumption of homogeneity of tissue. The reason for decrement of Sco2 with increasing postconceptional age is that cerebral Hb oxygen consumption increases with advance of postconceptional age, and this leads to a decrease in venous Hb oxygen saturation. The lower values of cerebral oxygen consumption in neonates than in older children is likely to be due to changes in the structural complexity and functional activity of the brain that occur across the range of gestational ages (25–27). Another reason is that venous structural change in the brain surface occurs in this period. The ratio of venous to arterial vessels may increase, and this would lead to an increase in cerebral content of deoxyHb and therefore to decrease in Sco2.
In this study, we noninvasively estimated values of CBV in neonates using a TRS system. Brazy et al. (1,28) monitored changes in CBV in infants, but quantification of CBV has not been possible. Methods for calculating CBV using oxyHb as a tracer with continuous-wave NIRS have been reported (29,30). Moreover, without changing oxyHb, CBV has been measured using indocyanine green (ICG) with spatially resolved NIRS (31). However, to our knowledge, there have been no studies in which CBV in infants was estimated by using a TRS system without changes in oxyHb or without using ICG injection. The mean value obtained in the neonates in the present study, 2.31 ± 0.56 mL/100 g, is similar to the values estimated in infants by using the continuous-wave NIRS method with changes in arterial Hb saturation (2.2–3.0 mL/100 g) (29) and with changes in Pco2 (3.7 mL/100 g) (30). These values in infants all are lower than those estimated in human adults using singe photon emission computed tomography (4.8 ± 0.4 mL/100 g) (32) and using positron emission tomography (4.7 ± 1.1 mL/100 g) (33). The reason for smaller values of CBV in neonates than in adults is that regional CBV is smaller in white cerebral matter than in gray cerebral matter, and the relatively low mean CBV may reflect a relative preponderance of white matter compared with that in the adult brain (29). In this study, the values of CBV increased with advance of postconceptional age. This relationship between postconceptional age and CBV is based on the results of an anatomic study of cerebral blood vessels showing that the percentage of blood vessel area in gray matter and white matter increased as a function of gestational age (34). Indeed, the CBV value estimated by using spatially resolved NIRS with ICG (1.72 ± 0.76 mL/100 g) was smaller than that obtained in our study, because the gestational age of the subjects in that study (mean gestational age 28 wk; mean postnatal age 6 d) was less than that of our patients (31).
DPFs at 761, 795, and 835 nm were estimated to be 4.58 ± 0.41, 4.64 ± 0.46, and 4.31 ± 0.42, respectively. Various experimental techniques have been used to determine DPF in infants. These are based on measurements of absorption of light by time-of-flight spectroscopy (35,36), phase-resolved spectroscopy (26,37), or the water peak method (8). The mean value of DPFs estimated by using time-of-flight spectroscopy at 783 nm in postmortem infants has been reported to be 4.39 ± 0.28 (n = 6) (35) and 3.85 ± 0.57 (n = 10) (36). Duncan et al. (26,37) measured DPFs in a group of 35 infants using phase-resolved spectroscopy and calculated the mean values to be 5.11 ± 0.48 at 744 nm and 4.67 ± 0.65 at 832 nm. DPFs that were calculated by the water peak method at 730 nm and 830 nm were 4.66 ± 1.01 and 3.91 ± 0.75, respectively (8). The DPF values obtained in this study are similar to those obtained in the group of infants using phase-resolved spectroscopy.
The sensitivity and the reliability of our TRS method were previously assessed by using an in vitro model and a piglet hypoxia model. In the in vitro study, the use of intralipids and a blood phantom showed that qualitative measurements of Hb concentrations and of oxygen saturation could be made under the same conditions as those in an in vivo study. In the piglet hypoxia model, the mean Sco2 value at normoxia was calculated to be 62%, and the contributions of arterial blood and venous blood were estimated to be 41 and 59%, respectively (38). The ratio of the contribution of arterial blood to that of venous blood obtained in the study using a piglet hypoxia model is almost the same as the ratio reported by Brun et al. (39) and by Kusaka et al. (40). The results of the present study demonstrated that our TRS method can be used to monitor Sco2 and CBV. However, the number of such neonates in this study was too small for us to reach any definite conclusion, and further study is required.
In conclusion, the results of this study confirm that the new TRS is a practical method for measurements of μ's, Sco2, CBV, and DPF in neonates at the bedside in an intensive care unit.
Abbreviations
- CBV:
-
cerebral blood volume
- DPF:
-
differential pathlength factor
- ICG:
-
indocyanine green
- NIRS:
-
near-infrared spectroscopy
- PLP:
-
picosecond light pulser
- Sco2:
-
cerebral Hb oxygen saturation
- Spo2:
-
oxygen saturation by pulse oximeter
- TRS:
-
time-resolved spectroscopy
- μa:
-
light absorption coefficient
- μ's:
-
light-reduced scattering coefficient
References
Brazy JE, Lewis DV, Mitnick MH, Jobsis van der Vliet FF 1985 Noninvasive monitoring of cerebral oxygenation in preterm infants: preliminary observations. Pediatrics 75: 217–225
Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EO 1986 Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2: 1063–1066
Edwards AD, Wyatt JS, Richardson C, Delpy DT, Cope M, Reynolds EO 1988 Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet 2: 770–771
Liem KD, Hopman JC, Oeseburg B, de Haan AF, Festen C, Kollee LA 1995 Cerebral oxygenation and hemodynamics during induction of extracorporeal membrane oxygenation as investigated by near infrared spectrophotometry. Pediatrics 95: 555–561
Pryds O, Greisen G, Skov LL, Friis-Hansen B 1990 Carbon dioxide-related changes in cerebral blood volume and cerebral blood flow in mechanically ventilated preterm neonates: comparison of near infrared spectrophotometry and 133Xenon clearance. Pediatr Res 27: 445–449
van Bel F, Dorrepaal CA, Benders MJ, Zeeuwe PE, van de Bor M, Berger HM 1993 Changes in cerebral hemodynamics and oxygenation in the first 24 hours after birth asphyxia. Pediatrics 92: 365–372
Tyszczuk L, Meek J, Elwell C, Wyatt JS 1998 Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics 102: 337–341
Cooper CE, Elwell CE, Meek JH, Matcher SJ, Wyatt JS, Cope M, Delpy DT 1996 The noninvasive measurement of absolute cerebral deoxyhemoglobin concentration and mean optical path length in the neonatal brain by second derivative near infrared spectroscopy. Pediatr Res 39: 32–38
Kusaka T, Isobe K, Kawada K, Ohtaki Y, Itoh S, Hirao K, Onishi S 1998 Postnatal changes in the cerebral oxygenation in normal and asphyxiated neonates. Proc SPIE Int Soc Opt Eng 3194: 92–102
Isobe K, Kusaka T, Fujikawa Y, Kondo M, Kawada K, Yasuda S, Itoh S, Hirao K, Onishi S 2000 Changes in cerebral hemoglobin concentration and oxygen saturation immediately after birth in the human neonate using full-spectrum near infrared spectroscopy. J Biomed Opt 5: 283–286
Isobe K, Kusaka T, Fujikawa Y, Okubo K, Nagano K, Yasuda S, Kondo M, Itoh S, Hirao K, Onishi S 2002 Measurement of cerebral oxygenation in neonates after vaginal delivery and cesarean section using full-spectrum near infrared spectroscopy. Comp Biochem Physiol A Mol Integr Physiol 132: 133–138
Naulaers G, Morren G, Van Huffel S, Casaer P, Devlieger H 2002 Cerebral tissue oxygenation index in very premature infants. Arch Dis Child Fetal Neonatal Ed 87: F189–F192
Liebert A, Wabnitz H, Grosenick D, Macdonald R 2003 Fiber dispersion in time domain measurements compromising the accuracy of determination of optical properties of strongly scattering media. J Biomed Opt 8: 512–516
Kusaka T, Hisamatsu Y, Kawada K, Okubo K, Okada H, Namba M, Imai T, Isobe K, Itoh S 2003 Measurement of cerebral optical pathlength as a function of oxygenation using near-infrared time-resolved spectroscopy in a piglet model of hypoxia. Opt Rev 10: 466–469
Oda M, Yamashita Y, Nakano T, Suzuki A, Shimizu K, Hirano I, Shimomura F, Ohmae E, Suzuki T, Tsuchiya Y 1999 Near infrared time-resolved spectroscopy system for tissue oxygenation monitor. Proc SPIE Int Soc Opt Eng 3597: 611–617
Patterson MS, Chance B, Wilson B 1989 Time resolved reflectance and transmittance for the non-invasive measurement of optical properties. Appl Opt 289: 2331–2336
Bolin PF, Preuss LE, Taylor RC, Ference RJ 1989 Refractive index of some mammalian tissues using a fiber optic cladding method. Appl Opt 28: 2297–2303
Delpy DT, Cope M, van der Zee P, Arridge S, Wray S, Wyatt J 1988 Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol 33: 1433–1442
Anthony MY, Evans DH, Levene MI 1991 Cyclical variations in cerebral blood flow velocity. Arch Dis Child 66: 12–16
Taga G, Konishi Y, Maki A, Tachibana T, Fujiwara M, Koizumi H 2000 Spontaneous oscillation of oxy- and deoxy-hemoglobin changes with a phase difference throughout the occipital cortex of newborn infants observed using non-invasive optical topography. Neurosci Lett 282: 101–104
Zhang G, Katz A, Alfano RR, Kofinas AD, Kofinas DA, Stubblefield PG, Rosenfeld W, Beyer D, Maulik D, Stankovic MR 2000 Brain perfusion monitoring with frequency-domain and continuous-wave near-infrared spectroscopy: a cross-correlation study in newborn piglets. Phys Med Biol 45: 3143–3158
Yamashita Y, Oda M, Naruse H, Tamura M 1996 In vivo measurement of reduced scattering and absorption coefficients of living tissue using time-resolved spectroscopy. In: Alfano RR, Fujimoto GJ (eds) OSA TOPS on Advances in Optical Imaging and Photon Migration, Optical Society of America, Washington, D.C., Vol 2. pp 387–390
Dani C, Bertini G, Reali MF, Tronchin M, Wiechmann L, Martelli E, Rubaltelli FF 2000 Brain hemodynamic changes in preterm infants after maintenance dose caffeine and aminophylline treatment. Biol Neonate 78: 27–32
Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC 2000 Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology 93: 947–953
Yoxall CW, Weindling AM 1998 Measurement of cerebral oxygen consumption in the human neonate using near infrared spectroscopy: cerebral oxygen consumption increases with advancing gestational age. Pediatr Res 44: 283–290
Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, Cope M, Delpy DT 1996 Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res 39: 889–894
Altman DI, Perlman JM, Volpe JJ, Powers WJ 1993 Cerebral oxygen metabolism in newborns. Pediatrics 92: 99–104
Brazy JE, Lewis DV 1986 Changes in cerebral blood volume and cytochrome aa3 during hypertensive peaks in preterm infants. J Pediatr 108: 983–987
Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray S, Reynolds EO 1990 Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J Appl Physiol 68: 1086–1091
Brun NC, Greisen G 1994 Cerebrovascular responses to carbon dioxide as detected by near-infrared spectrophotometry: comparison of three different measures. Pediatr Res 36: 20–24
Leung TS, Aladangady N, Elwell CE, Delpy DT, Costeloe K 2004 A new method for the measurement of cerebral blood volume and total circulating blood volume using near infrared spatially resolved spectroscopy and indocyanine green: application and validation in neonates. Pediatr Res 55: 134–141
Sakai F, Nakazawa K, Tazaki Y, Ishii K, Hino H, Igarashi H, Kanda T 1985 Regional cerebral blood volume and hematocrit measured in normal human volunteers by single-photon emission computed tomography. J Cereb Blood Flow Metab 5: 207–213
Powers WJ, Grubb RL Jr, Darriet D, Raichle ME 1985 Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab 5: 600–608
Ballabh P, Braun A, Nedergaard M 2004 Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res 56: 117–124
Wyatt JS, Cope M, Delpy DT, van der Zee P, Arridge S, Edwards AD, Reynolds EO 1990 Measurement of optical path length for cerebral near-infrared spectroscopy in newborn infants. Dev Neurosci 12: 140–144
van der Zee P, Cope M, Arridge SR, Essenpreis M, Potter LA, Edwards AD, Wyatt JS, McCormick DC, Roth SC, Reynolds EO 1992 Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 316: 143–153
Duncan A, Meek JH, Clemence M, Elwell CE, Tyszczuk L, Cope M, Delpy DT 1995 Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys Med Biol 40: 295–304
Ijichi S, Kusaka T, Isobe K, Islam F, Okubo K, Okada H, Namba M, Kawada K, Imai T, Itoh S 2005 Quantification of cerebral hemoglobin as a function of oxygenation using near-infrared time-resolved spectroscopy in a piglet model of hypoxia. J Biomed Opt 10: 24026
Brun NC, Moen A, Borch K, Saugstad OD, Greisen G 1997 Near-infrared monitoring of cerebral tissue oxygen saturation and blood volume in newborn piglets. Am J Physiol 273: H682–H686
Kusaka T, Isobe K, Nagano K, Okubo K, Yasuda S, Kondo M, Itoh S, Hirao K, Onishi S 2002 Quantification of cerebral oxygenation by full-spectrum near-infrared spectroscopy using a two-point method. Comp Biochem Physiol A Mol Integr Physiol 132: 121–132
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants-in-aid for scientific research 17390307, 16591075, 15591158, and 15591159, from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Rights and permissions
About this article
Cite this article
Ijichi, S., Kusaka, T., Isobe, K. et al. Developmental Changes of Optical Properties in Neonates Determined by Near-Infrared Time-Resolved Spectroscopy. Pediatr Res 58, 568–573 (2005). https://doi.org/10.1203/01.PDR.0000175638.98041.0E
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000175638.98041.0E
This article is cited by
-
Impact of hydrogen gas inhalation during therapeutic hypothermia on cerebral hemodynamics and oxygenation in the asphyxiated piglet
Scientific Reports (2023)
-
Spatial–Temporal Oxygenation Mapping Using a Near-Infrared Optical Scanner: Towards Peripheral Vascular Imaging
Annals of Biomedical Engineering (2023)
-
Relationship of cerebral blood volume with arterial and venous flow velocities in extremely low-birth-weight infants
European Journal of Pediatrics (2023)
-
Cerebral hemodynamic response during the resuscitation period after hypoxic-ischemic insult predicts brain injury on day 5 after insult in newborn piglets
Scientific Reports (2022)
-
Foetal growth, birth transition, enteral nutrition and brain light scattering
Scientific Reports (2021)