Abstract
Thermally stable and photosensitive polymers (TSPSPs), such as photosensitive polyimides (PSPIs) and polybenzoxazoles (PSPBOs), have been widely developed in recent years for their applications in electronic packaging (for example, buffer coating, passivation layers, and insulation layers) and redistribution in microelectronics due to their excellent thermal and reasonable dielectric properties. This review describes recent advances in TSPSPs during the last decade and focuses on related design concepts. Following a brief introduction of the basic patterning procedure, various chemistries used for the design of TSPSPs are highlighted along with two major patterning methodologies: positive- and negative-working PSPIs/PSPBOs. Furthermore, new applications of TSPSPs (for example, low dielectric materials, high refractive index lenses, resists for indium tin oxide patterning, and π-conjugated polymers for organic-field effect transistors) are introduced.
Similar content being viewed by others
Introduction
Our lifestyles have changed completely as a result of significant advances made in the field of electronics since 1970. In particular, the computer industry based on the transistor led to the innovations in personal computers, portable phones, transportation systems, and medical equipment, and the introduction of computer-guided robots in factories. Currently, rapid miniaturization and multi-functionalization processes are occurring in the semiconductor industry to keep pace with progress made in the information society. To increase memory capacities, the minimum pitch resolution will reach ~10 nm through the use of a combination of chemically amplified resists and extreme ultraviolet lithography technologies. Simultaneously, high-density electronic packaging is evolving from the use of two-dimensional packaging to three-dimensional packaging that is characterized by embedded electronic components and by direct connections between them.
Thermally stable and photosensitive polymers (TSPSPs) such as photosensitive polyimides (PSPIs) and polybenzoxazoles (PSPBOs) have played a central role in electronic packaging and have been widely used as buffer coatings, passivation layers, and insulation layers for redistribution in microelectronics owing to their excellent thermal and reasonable dielectric properties.1, 2, 3, 4 They simplify patterning processing by eliminating the use of a photoresist, but TSPSPs must have the following two distinctive attributes: photopatternable properties to delineate patterns and adequate durability required for final products. High levels of sensitivity, contrast and resolution, transparency in the visible region, and a long shelf life are necessary as photopatternable properties. Moreover, high levels of thermal stability, mechanical strength, dimension stability, electrical properties, adhesion, and chemical resistance are essential qualities that guarantee the durability of films. Several review papers have been published on the development of PSPIs and PSPBOs.5, 6, 7, 8, 9 Thus, the present review article describes recent progress made in TSPSPs over the last decade. A basic patterning procedure is briefly introduced, and various chemistries of TSPSPs are then described along with the following two major patterning methodologies: positive- and negative-working PSPIs/PSPBOs. Furthermore, new applications of TSPSPs are introduced.
Photolithographic process
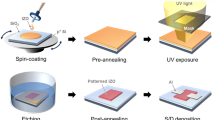
A schematic representation of the photolithographic process sequence is shown in Figure 1. A TSPSP solution is spin-cast on a substrate. The resulting film is prebaked on a hotplate and is exposed to UV light through a mask, whereby wavelengths of 436 nm (g-line), 405 nm (h-line), or 365 nm (i-line) generated from an ultrahigh pressure mercury lamp are used as UV light sources. Photo-irradiation induces the chemical reaction of matrix polymers or photosensitive compounds via crosslinking, chain-scission, deprotection, rearrangement, and cyclization upon post-exposure baking (PEB) when necessary. The following development process with an organic solvent or an alkaline solution produces a pattern. The exposed area becomes more soluble in the developing solvent than in the unexposed area, producing a positive-tone image of the mask (a); otherwise, the exposed area is rendered less soluble, forming a negative-tone image of the mask (b).
Typical pattern formation
For the design of TSPSPs, a polarity change method whereby photosensitive compounds such as diazonaphthoquinones (DNQs) and photoacid generators (PAGs) are incorporated into matrix polymers is widely employed. The role of these additives is described as follows.
Dissolution inhibitor: DNQs
DNQs are widely used as dissolution inhibitors (DIs) for classical positive-working photoresists during microlithography. For example, hydrophobic DNQs act as DIs for the aqueous base development of a novolac resin. Upon exposure to UV light, DNQs are transformed into indenecarboxylic acid derivatives (Scheme 1), accelerating dissolution in aqueous alkaline solutions and thereby forming positive images.10
Chemical amplification system
In a chemical amplification (CA) system, the presence of a single photochemical spurs a cascade of subsequent chemical transformations in a resist film whereby acid species generated from PAG catalyze numerous chemical reactions combined with the PEB of exposed film. CA changes the structure and physical properties of a polymeric material. Scheme 2 shows a typical schematic illustration of CA process. Phenol groups of poly(4-hydroxystyrene) (PHOST) are protected with acid-labile tert-butoxycarbonyl (t-Boc) groups. Upon exposure to UV light followed by PEB treatment at ~100 °C for several minutes, the hydrophobic t-Boc-PHOST is converted into an alkaline-soluble PHOST through its reaction with a photogenerated acid, releasing carbon dioxide, isobutene, and a proton.11
Negative-type PSPIS
Many papers have been published on the imidization of poly(amide ester) (PAE).6, 12, 13, 14, 15 It has been found that the poly(amic acid) (PAA) precursor undergoes imidization reactions at much lower temperatures than the PAE precursor due to the effects of solvents such as 1-methyl-2-pyrrolidone (NMP). NMP strongly complexes with carboxylic groups of the PAA through hydrogen bonds. Thus, a significant amount of NMP remains in the film even at high temperatures, producing a highly mobile-reacting species for curing at a low temperature. Recently, imidization reactions in thin films of the PAE-type PSPI were investigated in a temperature range of 50–450 °C by temperature-dependent rapid-scan in situ FT-IR spectroscopy, thermo-ellipsometric analysis, and thermogravimetric analysis coupled with evolved gas analysis.16 The relationship between the imidization of an unexposed PSPI precursor sample and UV-exposed samples irradiated with 250 and 1000 mJ cm−2 doses is shown in Figure 2. The imidization of non-crosslinked PAE was completed at 250 °C. By contrast, UV-exposed PAE required raising the temperature to above 340 °C to achieve full imidization depending on the degree of crosslinking because the cleave-out of the crosslinker is required for complete imidization.
Unexposed and exposed photosensitive polyimide (PSPI) films measured at 5 °C min−1 via in situ FT-IR spectroscopy and temperature-dependent spectroscopic ellipsometry. Reproduced with permission from ref. 16. Copyright 2017 Elsevier.
A multichip package through which the thickness of a silicon wafer is made thinner than 50 μm has been utilized to increase memory-density levels. A high-temperature cyclization process induces a high level of thermal stress in the silicon wafer, causing warping. Therefore, a significant decrease in the cyclization temperatures of PAAs and poly(hydroxyl amide)s (PHAs) as precursors of polyimides (PIs) and polybenzoxazoles (PBOs), respectively, is necessary for the versatile development of PSPI and PSPBO systems, producing more useful applications for the microelectronics industry. Although PAAs with hydrophilic carboxylic acids seem to constitute good candidates as PSPI precursors, the dissolution rate of PAA in a 2.38 wt% aqueous tetramethylammonium hydroxide (TMAH(aq)) solution is very high and it is difficult to obtain a sufficient level of dissolution contrast between unexposed and exposed areas in the presence of DIs due to the high acidity of carboxylic acids in PAAs. Ueda and colleagues17 reported a negative-type PSPI based on a PAA and photobase generator (PBG), {[(4,5-dimethoxy-2-nitrobenzyl)oxy]carbonyl}-2,6-dimethyl piperidine (DNCDP), in which a photochemically generated base catalyzes the thermal cyclization of PAAs to PIs (Scheme 3). Consequently, the exposed area following PEB (160 °C) becomes insoluble in a 2.38 wt% TMAH(aq) solution and produces a negative pattern. This patterning process is novel and versatile, enabling the direct transformation of PAAs to corresponding PIs. Moreover, the low-temperature imidization (200 °C) of PAAs to PIs was accomplished using a photogenerated base catalyst. However, a large amount of DNCDP (PAA/DNCDP=80/20, wt%) is needed to ensure a good pattern due to the low sensitivity of DNCDP.
Recently, Sakayori and colleagues18, 19 developed highly sensitive PBGs from o-hydroxy-trans-cinnamic acid derivatives, in which the photosensitivity of DNCDP was found to be lower than that of PBG-1 (Figure 3). The photodegradation rate of PBGs was studied as is shown in Figure 4. Characteristic sensitivity curves of the PBGs clearly show that PBG-3 is a highly sensitive PBG.
Chemical structures of new photobase generators (PBGs). Reproduced with modifications with permission from ref. 19. Copyright 2011 The Society of Photopolymer Science and Technology (SPST).
Conversion rates of photobase generators (PBGs) against exposure doses under a light intensity of 1.4 mW cm−2 at 365 nm. Reproduced with modifications with permission from ref. 19. Copyright 2011 The Society of Photopolymer Science and Technology (SPST).
Alkaline-developable soluble polyhydroxyimide (PHI) serves as a good candidate as a polymer matrix for eliminating the high-temperature-curing step of the patterning process.20, 21, 22, 23 Using a similar strategy, an alkaline-developable, chemically amplified, negative-type PSPI based on PHI, a crosslinker, and a PAG was developed.24 The PHI was prepared from cyclobutanetetracarboxylic dianhydride and 4,4′-(hexafluoroisopropylidene)bis(2-aminophenol) (6FAP). The chemically amplified PSPI consists of PHI, 4,4′-methylenebis[2,6-bis(hydroxymethyl)phenol] (MBHP) as a crosslinker, and (5-propylsulfonyloxyimino-5H-thiophen-2-ylidene)-(2-methylphenyl)acetonitrile (PTMA) as a PAG. The corresponding patterning process is shown in Scheme 4. The chemically amplified PSPI consisting of PHI (70 wt%), MBHP (20 wt%), and PTMA (10 wt%) was prebaked at 80 °C for 3 min, exposed to irradiation at a wavelength of 365 nm (i-line) with various exposure doses, post-exposure baked at 140 °C for 1 min, and developed in a 2.38 wt% TMAH(aq) solution for 3 s at room temperature. The resulting resist showed a high level of sensitivity (D0.5) of 5.9 mJ cm−2 and a strong contrast (γ0.5) level of 3.9, producing a clear negative-tone line and space pattern with a 6-μm resolution. Moreover, the PSPI exhibited the low dielectric constant (ɛ) of 2.54.
Positive-type PSPIs
Positive-type PSPIs generate much finer images than negative-type PSPIs by preventing the swelling of patterns during development and by forming a V-type pattern, which is desirable for wire-bonding processes. Furthermore, environmentally friendly fabrication and patterning processes are increasingly desired in microelectronics in recent years. Therefore, an alkaline aqueous solution is preferable as a developer in place of organic developers. Tomikawa et al.25 reported a positive-type PSPI developed from partially esterified PAAs. The esterification of PAA using dimethylformamide diethyl acetal (DMFDEA) was investigated (Scheme 5). Figure 5 shows the effects of the amounts of DMFDEA to PAA on esterification. The extent of the esterification reaches 70% when using an equivalent of DMFDEA, during which imidization also occurs at ~25%. The partial esterification of PAA was carried out by reacting PAA (Mn=9000) with DMFDEA (93 mol%). PSPI was formulated from the partially esterified PAA and DNQ, as is shown in Figure 6, followed by spin-coating on a silicon wafer and prebaking (PB) at 120 °C for 3 min. The exposed film was developed in a 2.38 wt% TMAH(aq) solution and was heated at 320 °C for 1 h. A clear positive image was produced (Figure 6). This partial esterification from DMFDEA is unique and applicable to various PAAs. Another approach using crosslinking and de-crosslinking reactions of PAAs has been developed, that is, an alkaline-developable, chemically amplified positive-type PSPI based on a PAA, a crosslinker, 1,1-tris(4-(2-(vinyloxy)ethoxy)phenyl)ethane (TVPE), a PAG, PTMA, and a thermobase generator (TBG), t-butyl-2,6-dimethylpiperidine-1-carboxylate (BDPC).26 The development of latent base catalysts is desirable to accomplish the low-temperature imidization of PAAs. Latent catalysts are stable compounds under ambient conditions that function only following external stimulation (for example, heating or photo-irradiation). The patterning process using this PSPI is shown in Scheme 6.
Dimethylformamide diethyl acetal (DMFDEA) volume dependence on esterification and imidization. Reproduced with permission from ref. 25. Copyright 2009 The Society of Polymer Science, Japan.
Composition of positive photosensitive polyimide (PSPI) and an SEM image (10-μm line and space). Reproduced with permission from ref. 25. Copyright 2009 The Society of Polymer Science, Japan.
The PAA (69 wt%) solution containing TVPE (21 wt%), PTMA (3 wt%), and BDPC (7 wt%) was spin-coated on a silicon wafer followed by PB at 130 °C for 3 min, which induced a crosslinking reaction between the carboxylic acid of PAA and TVPE. The film was then exposed to 50 mJ cm−2 of g-line (436 nm) followed by PEB at 90 °C for 2 min, resulting in the acid catalytically de-crosslinking of PAA by the photogenerated propane sulfonic acid. The exposed and post-baked film was then developed in a 1.69 wt% TMAH(aq) solution to produce a positive image of an 8-μm line and space pattern that was fully converted into a corresponding PI pattern by heating at 200 °C for 1 h with the aid of a base generated from BDPC (Figure 7). Furthermore, low-temperature imidization was accomplished.
SEM image of polyimide (PI) pattern cured at 200 °C for 1 h. Reproduced with permission from ref. 26. Copyright 2009 Wiley Periodicals.
The dissolution rate of PAAs to a 2.38 wt% TMAH(aq) solution can be controlled from the balancing of a hydrophilic carboxylic acid and hydrophobic aromatic unit in PAAs.27 An alkaline-developable and chemically amplified PSPI was designed, whereby highly fluorinated PAA (FPAA) obtained from 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6FDA) and 2,2-bis[4-(aminophenoxy)phenyl]-hexafluoropropane was used to reduce the dissolution rate in a TMAH(aq) solution. The PSPI was formulated from PAA, 9,9-bis[4-(tert-butoxycarbonylmethyloxy)phenyl]fluorene (TBMPF) as a DI, and PTMA (Scheme 7).28
The PSPI film consisting of FPAA (84 wt%), TBMPF (8 wt%), and PTMA (8 wt%) was prebaked at 110 °C for 2 min, exposed to 436 nm (g-line) and developed in a 2.38 wt% TMAH(aq)/5 wt% isopropanol (iPrOH) solution. The resist exhibited a high sensitivity level of 45 mJ cm−2 and an excellent contrast of 10, producing a clear positive image of 6-μm line and space patterns. Using a similar approach, an alkaline-developable PSPI based on FPAA prepared from 6FDA and 4,4′-oxydianile (ODA) and a fluorinated diazonaphthoquinone (FDNQ) was developed.29 The corresponding photolithographic process is shown in Scheme 8.
The film prepared from FPAA and FDNQ (25 wt% to FPAA) was prebaked, producing a FDNQ-rich surface, which was confirmed by measurements of the contact angle of water on the film. As shown in Figure 8, the contact angles gradually increase with increasing PB temperatures, suggesting that the segregation and migration of FDNQ at the surface are promoted during PB. The film exposed to the i-line and developed in a 2.38 wt% TMAH(aq) solution exhibited a high sensitivity level of 45 mJ cm−2 and an excellent contrast of 10. Unique patterns of PI generated while using PSPBO as the top layer were demonstrated.30 The patterning process of this two-layered system is shown in Scheme 9. The two-layered system consisted of a thick PAA base layer and a thin upper PSPBO layer consisting of PHA, TBMPF, and PTMA. The thin layer (200 nm) of PSPBO containing PHA (74 wt%), TBMPF (22 wt%), and PTMA (4 wt%) formed on the thick PAA film (1.8 μm). The film was prebaked at 120 °C for 2 min and was exposed to 100 mJ cm−2 of the i-line to generate propane sulfonic acid from PTMA. Upon PEB treating (130 °C for 2 min) the film, the acid deprotected the tert-butyl ester of TBMPF. The film was finally developed in a 2.38 wt% TMAH(aq)/5 wt% iPrOH solution at 25 °C for 2 s. A clear positive image of a 4-μm line and space pattern was produced. Finally, thermal treatments of the film at 250 °C and 350 °C for 30 min produced a PBO/PI film (Figure 9).
Contact angles of films after prebaking (PB). Films consisting of fluorinated poly(amic acid) (FPAA) and fluorinated diazonaphthoquinone (FDNQ) (25 wt% to FPAA) were used. The PB time was set for 2 min. Reproduced with modifications with permission from ref. 29. Copyright 2013 The Society of Photopolymer Science and Technology (SPST).
Cross-section views of the two-layered film observed by SEM. (a) Polybenzoxazoles (PSPBOs)/poly(amic acid) (PAA) film. (b) PBO/polyimide (PI) film after curing at 250 °C for 30 min and then at 350 °C for 30 min under nitrogen. Reproduced with permission from ref. 30. Copyright 2010 Elsevier.
The described method is beneficial in its broad applicability to various PAA layers. The PI-patterning process described above is interesting but still involves the application of two steps to form a bilayer. To enable a more efficient and versatile process, simple PI-pattern formations should be designed. Recently, a more straightforward PI-pattern formation involving FPAA, PAA, and FDNQ was reported.31 Scheme 10 presents the related photolithographic process. When PSPI consisting of PAA (85 wt%), FPAA (15 wt%), and FDNQ (25 wt% to polymers) was prebaked at 130 °C for 2 min, two phase-separated layers (shown in Figure 10) including a thin upper layer of FPAA-containing FDNQ and a thick bottom layer of PAA formed spontaneously due to the lower surface tension of the fluorinated compounds (FPAA and FDNQ) compared to that of PAA. The film was then exposed to 100 mJ cm−2 of the i-line, producing an indenecarboxylic acid, which was soluble in an aqueous base solution. In turn, the dissolution rate of the exposed area in the 0.238 wt% TMAH(aq) solution increased and a clear positive pattern was obtained from a 6-μm feature on a 1-μm thick film. The PB process affects sensitivity levels and pattern shapes. PB effects of the residual solvent in the film and of DNQ decompositions on the lithographic performance of a positive-type PSPI were investigated.32 A PSPI based on partially esterified PAA and DNQ was formulated in γ-butyrolactone (GBL). After PB at ~110–130 °C for several minutes, residual solvent levels reached 9–14 wt% due to the high boiling point (203 °C) of GBL. Table 1 presents a summary of the decomposition results of DNQ during PB. The decomposition of DNQ was ~10% at 110 and 120 °C, but DNQ decomposed rapidly at 130 °C. On the basis of these data, a suitable PB approach for this positive-type PSPI was determined to involve applying 120 °C for 4 min.
Cross-sectional SEM image of a patterned poly(amic acid) (PAA) film (PAA (85 wt%), fluorinated PAA (FPAA) (15 wt%) and fluorinated diazonaphthoquinone (FDNQ) (25 wt% to polymers)). The prebaking (PB) condition was 130 °C for 2 min. Reproduced with permission from ref. 31. Copyright 2013 The Royal Society of Chemistry.
A flexible-printed circuit board (FPC) has an important role in microelectronic devices and recently introduced requirements include low levels of stiffness and resistance to repeated bending stress, whereby a cover layer (CL) is used to protect the copper patterning of copper-clad laminates. A PSPI containing both siloxane and hydroxyl imide units for the CL was reported (Scheme 11).33 A PSPI incorporated with a DNQ into a PHI film exhibited high sensitivity to the i-line and exhibited a low modulus of 0.28 GPa. The PSPI described above exhibited poor adhesion to a copper surface. To solve this problem, several adhesion promoters, such as mercaptotriazole and mercaptothiadiazole derivatives, were used.34 A energy dispersive spectrometer-transmission electron microscope (EDS-TEM) analysis revealed the formation of a sulfur–copper bond that enhanced adhesion between the PSPI film and copper foil.
As described above, silicon wafers have been made thinner to achieve high-density packaging. Thus, PSPIs using PIs with low coefficients of thermal expansion (CTE) and with lower imidization temperatures are in high demand to prevent the warping of thinner silicon wafers. Poly(trans-1,4-cyclohexylenebiphenylene imide) obtained from 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA) and trans-1,4-cyclohexyldiamine (CHDA) exhibits high levels of thermal stability, a low ɛ value, high levels of transparency, and a low CTE value (10 p.p.m. K−1) due to its rigid and semialicyclic structure.35, 36 On the basis of this result, a negative-type PSPI based on semialicyclic PAA, poly(trans-1,4-cyclohexylenediphenylene amic acid), and DNCDP was developed as a next-generation insulation material (Scheme 12).37 The photosensitivity curve of the PSPI consisting of PAA (80 wt%) and DNCDP (20 wt%) films is shown in Figure 11. The PSPI exhibited an excellent sensitivity (D0) level of 70 mJ cm−2 and a high degree of contrast (γ0) of 10.3 with i-line exposure, indicating that a photogenerated 2,6-dimethylpiperidine (DMP) effectively catalyzes the imidization of PAA via PEB treatment, producing a large DC between exposed and unexposed areas.
Characteristic photosensitive curve for the poly(amic acid) (PAA)/{[(4,5-dimethoxy-2-nitrobenzyl)oxy]carbonyl}-2,6-dimethyl piperidine (DNCDP) (80/20 wt/wt) resist system with 1.7 lm-thick film. The prebake and post-exposure baking (PEB) were fixed at 100 °C for 5 min and at 190 °C for 5 min, respectively. Reproduced with permission from ref. 37. Copyright 2010 Wiley Periodicals.
This resist produced a clear negative image of a 6-μm feature in a 1.4-μm-thick film after the development in a 2.38 wt% TMAH(aq)/20 wt% solution of iPrOH. Finally, the partial PI film was converted into a complete PI film by thermal treatment at 250 °C for 1 h, whereby DMP catalyzed the imidization of PAA. The PSPI film exhibited a low CTE of 16 p.p.m. K−1. This PSPI system has several features; that is, (i) direct formulation of PSPI from the PAA polymerization solution, (ii) high sensitivity and contrast levels of 70 mJ cm−2 and 10.3, respectively, (iii) base-catalyzed low-temperature imidization at 250 °C, and (iv) a low CTE film of 16 p.p.m. K−1.
Few studies have been published on the formation of spherical nanoparticles, and the morphology of PAA films for PSPIs remains unknown. In 2012, Ogura et al.38 demonstrated that spherical nanoparticles after PB form during the preparation of PAA films derived from BPDA and ODA films for PSPIs. The molecular weights of PAA and the prebake temperature were found to strongly affect particle morphologies. The particle size distribution after PB at 100–150 °C for 5 min was narrow, in a range of 25–45 nm, and the mean particle diameter was measured to be 33 nm (Figure 12). This finding is very important for the development of PSPIs by providing deeper insight into the development processes that involve the use of organic solvents or aqueous base solutions.
Cross-sectional view of the film observed by SEM; 8k-PAA (BPDA/ODA): magnification: 4 × 104. Reproduced with permission from ref. 38. Copyright 2012 The Royal Society of Chemistry.
Negative-type PSPBOs
As noted above, chemically amplified-type PSPBOs based on PHA combined with DI and PAG exhibit high levels of sensitivity. A drawback of these PSPBOs, however, is that the Cu circuits corrode in microchips as a result of photogenerated acid from PAG. To remedy this problem, a new chemically amplified-type PSPBO with a different photoimage formation mechanism is now required for the next generation of PSPBO-resist systems. The use of PBG in place of PAG is the most straightforward approach. An alkaline-developable chemically amplified negative-type PSPBO based on PHA, an active ester-type crosslinker bis(p-nitrophenyl) suberoylate (BNPS), and DNCDP as a PBG has been developed to prevent the corrosion of Cu circuits in microchips.39 The patterning process of this PSPBO system is shown in Scheme 13.
The resulting PSPBO exhibited high levels of thermal stability, low levels of water absorption (<0.05%), low dielectric constants (ɛ=2.79) and excellent mechanical properties. PAG-induced Cu corrosion in the Cu-coated surface was observed based on the disappearance of its metallic sheen. The diffusion of photogenerated propane sulfonic acid in the polymer film induced this corrosion. By contrast, photogenerated DMP from PBG did not affect the corrosion process at all. These results clearly show that this resist formulation method using DNCDP and BNPS is very effective at addressing corrosion problems involving Cu circuits in microchips. p-Nitrophenol generated from BNPS after esterification, however, is not suited to this process because of its volatilization and resist coloration. Moreover, the quantum yield of DNCDP is low. To overcome these problems, an alkaline-developable and chemically amplified PSPBO resist consisting of PHA, 1,6-bis(vinyl sulfone)hexane (HBVS) as a vinyl sulfone-type crosslinker, and N-{[(4,5-methylenedioxy-2-α-methylnitrobenzyl)oxy]carbonyl}-2,6-dimethylpiperidine (MNCDP) as a novel PBG was developed.40 Base-catalyzed Michael addition reactions41 between the vinyl sulfone units and phenolic hydroxy groups of PHA did not yield any leaving groups, and an α-methyl group in the methylene unit of the benzyl group of DNCDP was expected to promote its hydrogen abstraction after irradiation for effective photobase generation.42, 43 The resulting polymer film had a low dielectric constant (ɛ: 2.78) and exhibited high levels of thermal stability, good mechanical properties, and low water absorption features. The novel patterning system did not corrode Cu circuits in semiconductor devices.
Positive-type PSPBOs
PSPBOs are generally formulated from PHA as a PBO precursor and from a DNQ.44, 45, 46, 47, 48 A phenol group of PHA affords adequate levels of solubility in a 2.38 wt% TMAH(aq) solution. Moreover, after the thermal cyclization of PHA, polar phenol groups, which increase the ɛ value of polymers, completely disappear. The PSPBO preparation method is very simple and merely involves the addition of DNQ to a solution of PHA. However, there are several drawbacks of PSPBOs. The sensitivity of these conventional PSPBOs is low (300 mJ cm−2) even with a 20–25 wt% loading of DNQ to the PHA. Furthermore, it is difficult to create thick resist patterns with these PSPBOs due to the strong absorbance of DNQ at 365 nm. To circumvent these problems, several approaches have been described in a previous review article.7 Ueda and colleagues developed a positive-type PSPBO based on a PHA, 9,9-bis(4-tert-butoxycarbonyloxyphenyl)fluorene (t-Boc BHF) as a DI and PTMA (Scheme 14).49
The PSPBO based on PHA (77 wt%), t-Boc BHF (20 wt%), and PTMA (3 wt%) exhibited a sensitivity of 34 mJ cm−2 and a contrast of 5.8 when exposed to i-line light and when developed in 2.38 wt% TMAH(aq)/5 wt% iPrOH. A clear positive image with 6-μm features and a 10-μm-thick pattern was produced and converted into a PBO pattern through heating at 350 °C for 1 h.
The commercially available PSPBO is based on PHA from 6FAP and 4,4′-oxybis(benzoic acid) owing to its high transparency at a wavelength of 365 nm. It is important to develop a novel photosensitive polymer with higher transparency for UV lithography. Absorption in the i-line region of the PHA is derived from a charge transfer (CT) type transition between benzoic acid and o-hydroxyamide rings.50 A sulfone group as a substitute for the hexafluoroisopropylidene group also lowers highest occupied molecular orbital (HOMO) levels and thus increases band gaps, which induce hypochromic (blue) shifts in absorption originating from HOMO→lowest unoccupied molecular orbital (LUMO) transitions.51 A study of a substitute for 4,4′-oxybis(benzoic acid) was also conducted. Time-dependent density functional theory (TD-DFT) calculations for the structure of o-hydroxybenzamide indicated that the introduction of substituents to the ortho position of the amide group shifted the λmax value to a shorter wavelength. From this study, PHA prepared from 6FAP and 2,2′-dimethyl-biphenyl-3,3′-dicarboxylic acid chloride was more transparent than that of PHA generated from 4,4′-oxybis(benzoyl chloride).52 A positive-type PSPBO developed from PHA, 1,3,5-tris-[(2-vinyloxy)ethoxy]benzene (TVEB), and PTMA was formulated, and the resulting polymer film exhibited high levels of photosensitivity and contrast levels of 32 mJ cm−2 and 7.2, respectively. Furthermore, a 6-μm-PSPBO film consisting of PHA, TVEB, and PTMA (15/3/2 by wt.) was exposed to the i-line at 80 mJ cm−2, was post-baked at 120 °C for 5 min and was developed in a 2.38 wt% TMAH(aq) solution, producing a clear positive image featuring a 6-μm line and space.
The low-temperature cyclization of PHA to PBO is also required to prevent the decomposition of thermally unstable organic components, such as glass-epoxy or glass-bis(maleimide)triazine resins, which can be found in a built-up or packaged board. Thus, a significant decrease in the cyclization temperature of PHA leads to broad applications of a PSPBO system. Strong acids act as effective catalysts for the low-temperature cyclization of PHA.53 In particular, PTMA lowered the cyclization temperature to 250 °C relative to levels of noncatalytic cyclization at 350 °C. On the basis of this finding, low-temperature curable PSPBOs consisting of PHA, DNQ, and PTMA as a thermoacid generator (TAG) or partially t-Boc-protected PHA and PTMA were developed.54, 55 These PSPBOs present several drawbacks (for example, the development of thick pattern formations in the former PSPBO and the need to introduce t-Boc groups into the latter PSPBO). A more convenient method that is widely applicable and provides an easy way for resist formulation should be developed. Ogura et al.56 reported an alkaline-developable chemically amplified positive-type PSPBO based on a PHA, t-Boc BHF, a TAG (isopropyl p-toluenesufonate, ITS) as a latent TAG, and PTMA. The patterning process and the resulting low-temperature cyclization pattern derived from PSPBO are shown in Scheme 15.
The PSPBO film consisting of PHA (73 wt%), t-Boc BHF (18 wt%), ITS (7.5 wt%), and PTMA (1.5 wt%) was prebaked at 100 °C for 5 min and exposed to 365 nm light (50 mJ cm−2) to produce propane sulfonic acid from PTMA. Upon PEB treatment of the PSPBO film (115 °C for 3 min), the propane sulfonic acid catalyzed the decomposition of ITS, producing propene and p-toluenesulfonic acid. These strong acids promoted deprotection reactions of t-Boc BHF to yield 9,9-bis(4-hydroxyphenyl) fluorene (BHF), a dissolution promoter. The exposed and baked film was then developed in a 2.38 wt% TMAH(aq) solution to form a positive image with a 3-μm feature in the 3-μm thick film. The sulfonic acids catalyzed the low-temperature cyclization of PHA into a PBO pattern at 250 °C for 10 min under nitrogen. This chemically amplified positive-type PSPBO is very sensitive and efficient compared to the standard PSPBO that requires the application of large exposure doses and high cyclization temperatures.
Another TSPSP approach not involving high-temperature treatment
To prevent warping of extremely thin silicon wafers resulting from high-temperature-thermal treatments, another approach involving the development of TSPSP, which does not require high-temperature-curing treatment, was developed. Engineering plastics exhibit high levels of thermal stability and excellent mechanical properties. When they are used as matrices for TSTSPs, conversion from the precursors of matrix polymers is not necessary. A negative-type chemically amplified photosensitive poly(ether ether sulfone) (PSPEES) based on poly(ether ether sulfone) (PEES), 4,4′-methylenebis[2,6-bis(methoxymethyl)phenol] (MBMP) and diphenylidonium 9,10-dimethoxyanthracene-2-sulfonate (DIAS) has been developed.57 The corresponding patterning process is shown in Scheme 16.
The PSPEES film (PEES/MBMP/DIAS=85/10/5, wt%) was prebaked at 80 °C for 1 min, exposed to 150 mJ cm−2 of the i-line and post-exposure baked at 170 °C for 3 min. The photogenerated acid produces benzylic carbocations from MBMP that react with aromatic rings of PEES. This reaction converts soluble PEES into an insoluble crosslinked polymer. After developing in DMAc for 15 s at room temperature, a clear pattern with a 4-μm feature was obtained. Using a similar strategy, a chemically amplified negative-type photosensitive poly(phenylene ether ketone) (PSPEK) was developed. Within it, a ketal-protected poly(phenylene ether ketone) (PEK), which is a soluble precursor of PEK, was used as a matrix polymer.58 These design concepts serve as a versatile, promising, and straightforward route for the formation of TSPSPs and are applied to most engineering plastics with an aromatic ring. Furthermore, the process does not involve high-temperature-curing treatment after development.
Reaction development patterning
Itatani and colleagues59 reported a unique pattern-forming process referred to as reaction development patterning (RDP) in 2001. The corresponding pattern formation mechanism is shown in Scheme 17.
(i) DNQ forms an indencarboxylic acid upon UV irradiation, creating an ammonium salt with ethanolamine; (ii) an acid-base reaction enhances the permeation of the developer into film in the exposed area; and (iii) the amine attacks an imide ring to produce a ring-opening product followed by depolymerization to form a positive pattern. RDP is a versatile method that can be applied to commercially available engineering plastics with functional groups for reacting with a nucleophile in a developer (for example, polycarbonates, polyarylates, and other PIs).9, 60, 61, 62 Chemical amplification has been successfully introduced to RDP with the addition of a small amount of PAG and an acid amplifier rather than large quantities of a photosensitive agent used for conventional RDP (Scheme 18).63
A photogenerated acid generated from PAG acts as a catalyst for the deprotection of the acid amplifier, yielding an acid that forms a salt with the developer. This salt in turn promotes the preferential infiltration of the developer. The presence of acid amplifiers with sulfonate groups reduces levels of photosensitive agent from 30 to 5 wt% and increases sensitivity levels (125 mJ cm−2) relative to those of the conventional RDP (2000 mJ cm−2).
Application
Highly refractive index TSPSPs
Increasing assembly densities and decreasing device sizes are central to complementary metal oxide semiconductor image sensors used in mobile phones and digital cameras, in which microlenses are employed to collect light.64 An analog image is collected by the lenses, and a photodiode converts this analog image into a digital image. Microlenses are prepared using TSPSPs with a high refractive index (high-n), low levels of birefringence (Δn), high levels of thermal stability, and high levels of optical transparency. The Lorentz−Lorenz equation indicates that a high-n polymer can be produced through the introduction of substituents with a high molar refraction level and small molar volume. Therefore, the incorporation of aromatic rings, halogen atoms, except for fluorine, sulfur atoms, and metal atoms generates a high-n value.65, 66 Sulfur atoms and aromatic rings afford various polymers with high-n values of up to 1.7.67, 68, 69, 70, 71, 72, 73, 74 On the basis of this information, semialicyclic PAA (CHDA-3SDA) was successfully prepared via the polycondensation of 1,2,4,5-cyclohexanetetracarboxylic dianhydride (CHDA) with 4,4′-thiobis[(p-phenylenesulfanyl)aniline] (3SDA) and it exhibited high levels of affinity with silica-modified TiO2 nanoparticles. The PAA (CHDA-3SDA) combined with a 45 wt% load of TiO2 nanoparticles and DNCDP exhibited high levels of transparency (λcutoff=343 nm), high glass transition temperatures (Tg=237 °C), and good photolithographic characteristics (Scheme 19).75 A fine negative pattern with a resolution of ~4-μm was successfully printed onto the hybrid film. Furthermore, the cured PSPI-TiO2 hybrid film generated a refractive index of 1.8100 at 632.8 nm.
Tomikawa et al.76 also reported a high-n positive-type PSPI consisting of partially esterified PAA (10 wt%) obtained from 4,4′-oxydiphthalic anhydride (100 mol%), 6FAP (35 mol%), ODA (35 mol%), end capping agent 3-aminophenol (60 mol%), TiO2 surface-modified by an epoxysilane coupling agent (25 wt%), DNQ (3 wt%), and an UV absorbent (coumarin: 1 wt%). This PSPI achieved a high-n value of 1.78 at 633 nm with good transparency (>80% at 400 nm). The positive-type PSPI film was prebaked at 120 °C for 2 min, exposed to an i-line from 500 to 12 000 J m−2, and then developed in a 2.38% TMAH(aq) solution. The patterned wafer cured at 280 °C for 5 min produced a round profile. Effects of the plasticizer and UV absorbent on the developed pattern shape were investigated. Sulfonates as plasticizers failed to create a round profile, whereas coumarin as an UV absorbent produced a round profile. Saito et al.77 reported a highly refractive and photosensitive sulfur-containing PI derived from 4,4′-[p-thiobis(phenylenesulfanyl)] diphthalic anhydride (3SDEA) and 4,4′-thiodianiline, MBHP, and PTMA. The matrix polymer achieved a high-n value of 1.7452 and a low Δn value of 0.0081. The PSPI consisting of PI (84.8 wt%), MBHP (11.0 wt%), and PTMA (4.2 wt%) exhibited a high level of sensitivity (D0.5) of 36 mJ cm−2 and a strong contrast level (γ0.5) of 5.1 after PEB treatment at 110 °C for 3 min followed by its development in a 2.38 wt% TMAH(aq)/10 wt% IPA solution. A clearly negative pattern with an 8-μm feature was observed when a 1.3-μm thick film was used. A positive-type chemically amplified PSPHI consisting of partially imidized PHI obtained from 3SDEA and bis(3-amino-4-hydroxyphenyl)sulfone, TBMPF and PTMA was also observed as a high-n polymer.78 The PSPHI based on partially imidized PHI (80 wt%), TBMPF (16 wt%), and PTMA (4 wt%) generated a high-n value of 1.698 and a low Δn value of 0.0025 and exhibited a high sensitivity level of 25 mJ cm−2 and a contrast of 8.4.
Low dielectric constant TSPSPs
High-performance dielectrics exhibiting low dielectric constants (ɛ), low dissipation factors, low moisture uptake levels, and thermal stability (for example, a high Tg) must be developed for the next generation of microelectronic devices.79, 80 A typical engineering plastic, poly(2,6-dimethyl-1,4-phenylene ether) (PPE), constitutes a potential candidate due to its low ɛ (2.5) and low moisture uptake (< 0.05 wt%) levels. A negative-type photosensitive poly(phenylene ether) (PSPPE) with PPE as a polymer matrix was developed.81 A PSPEE consisting of PPE (73 wt%), MBMP (20 wt%), and DIAS (7 wt%) exhibited a high sensitivity (D0.5) level of 58 mJ cm−2 and a contrast level (γ0.5) of 9.5 at a film thickness of 1.5 μm when it was exposed to the i-line, post-exposure baked at 145 °C for 10 min, and developed in toluene. The cured PSPEE exhibited a low dielectric constant (ɛ=2.46) comparable to that of PPE, a higher Tg than that of PPE, and the same low water absorption (<0.05%) as that of PPE. A more efficient negative-working PSPPE was demonstrated, in which a new benzyl cation-type crosslinker, hex-1,6-ylenebis(oxy(2,4,6-tris(acetyloxymethyl)-3,5-dimethylbenzene)) (HOAD) was prepared.82 The cured PSPPE film at 220 °C for 1 h exhibited a low dielectric constant (ɛ=2.61), thermal stability (Tg=232 °C), and low levels of water absorption (<0.05%).
Novolac resists for indium tin oxide patterning
Patterned indium tin oxide (ITO) film acting as a transparent electrode plate has been used for a broad variety of displays, transistors, and solar cell fabrications. Although the novolac/DNQ resist is widely used for ITO patterning, its sensitivity is low and thick pattern formation is difficult due to the strong absorption of DNQ at the exposure wavelength. To remedy these problems, three-component chemically amplified novolac resists based on a novolac resin, a melamine crosslinker, and a PAG were developed and were found to be highly sensitive.83 A negative-type chemically amplified photosensitive resist consisting of novolac resin, MBMP, and PTMA has been proposed by Saito et al.84 Photogenerated propane sulfonic acid catalyzes the formation of benzylic carbocation species, which attack aromatic rings and hydroxyl groups to produce C- and O-alkylated polymers. The O-alkylated polymers thermodynamically rearrange into C-alkylated polymers through heating. These reactions produce a crosslinked polymer. The resist exhibited a high sensitivity (D0.5) level of 5.3 mJ cm−2 and a contrast (γ0.5) level of 3.5 at a film thickness of 1 μm when it was exposed to the i-line, post-exposure baked at 110 °C for 5 min, and developed in a 2.38 wt% TMAH(aq) solution. Furthermore, a 20-μm image formed in a 12-μm thick film using the resist based on novolac (90 wt%), MBMP (7 wt%), and PTMA (3 wt%).
Photosensitive poly(3-hexylthiophene) for OFET
π-Conjugated polymers (π-CPs) are expected to be accessible to optoelectronic applications due to their excellent processing at low-cost and large area fabrications.85 Among them, regioregular poly(3-hexylthiophene) (rr-P3HT) and related thiophene-based polymers have received considerable attention owing to their ease of preparation and processing and high-charge mobility. Therefore, P3HT has been used in a wide variety of electronic devices, such as organic solar cells,86 organic-field effect transistors (OFETs),87 and sensors.88 Pattern polymers are formed through a wide variety of techniques (for example, direct writing by scanning probe microscopy, nano-imprinting, micro-contact printing, ink-jet printing, dry-etch processing by laser ablation, and photolithography).89 A micro-patterned P3HT film is generally prepared via micro-contact printing with a poly(dimethylsiloxane) stamp.90 This method, however, involves several steps, resulting in low levels of productivity. To address this problem, direct patterning onto polythiophenes by photolithography should be developed. Holdcroft and colleagues91 reported direct patterning onto a P3HT film combined with a crosslinker by UV light irradiation, but related sensitivity levels were found to be very low. An efficient patterning method using poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]thiophene)92 and involving multi-stage patterning transfer processes via delamination using a poly(methyl methacrylate) dielectric film as a sacrificial layer has also been reported. Meanwhile, Qiu et al.93 developed photopatternable semiconducting/insulating polymer blends. Although this system has several merits (for example, low material costs, improved levels of environmental stability, and mechanical properties), the OFET properties observed were not sufficient for any practical applications. Ueda’s group reported chemically amplified photosensitive rr-P3HT with hexamethoxymethylmelamine (CYMEL) as a crosslinker and DIAS.94 The sensitivity of this resist was markedly improved compared to that of the chemically non-amplified photosensitive P3HT described above. Upon irradiation, a resist film based on rr-P3HT (70 wt%), CYMEL (20 wt%), and DIAS (10 wt%) was post-baked at 180 °C for 5 min, developed in THF, and rinsed with chloroform, producing a clear negative pattern with 4-μm features on a 100-nm thick film. As a disadvantage of this resist, large volumes of additives must be used (as much as 30 wt%), which may prevent the self-organizing of crystal structures of P3HT. To remedy this problem, a highly photosensitive P3HT with MBMP and DIAS was developed.95, 96 Upon photo-irradiation, photogenerated acids form benzyl cations of MBMP that react with not only thiophene rings but also with aromatic rings and with hydroxy groups of MBMP, yielded crosslinked polymers. A resist film based on P3HT (90 wt%), MBMP (5 wt%), and DIAS (5 wt%) was prebaked at 120 °C for 1 min, exposed to UV light for 5 min, post-baked at 170 °C for 30 s, and developed in chloroform, producing a clear negative pattern with 15-μm features on a 100-nm thick film. OFET devices of a patterned rr-P3HT formed from this resist with a bottom-contact configuration were constructed on an n-doped Si/SiO2 substrate and on gold-chromium bilayer source/drain electrodes through a simple spin-coating process involving the use of a 1,1,2,2-tetrachloroethane solution. Table 2 presents characteristics of the OFETs.
Although the OFET using the patterned rr-P3HT exhibited typical p-type characteristics after thermal treatment at 180 °C for 30 min, the charge carrier mobility and threshold voltage reached 0.026 cm2 V−1 s−1 and 7.5 V, respectively, suggesting that doping of rr-P3HT by the remaining PAG residue occurred (Figure 13a). To address this issue, further thermal and dedoping treatments were applied after development. The patterned film on SiO2 was treated as follows: heating at 200 °C for 30 min to decompose the PAG followed by treatment with a 1 wt% aqueous ammonia solution for 30 min at room temperature and then thermal annealing at 180 °C for 30 min. The resulting device exhibited high-charge carrier mobility in the saturation region of 0.092 cm2 V−1 s−1, a low threshold voltage of −1.6 V, and a high on/off current ratio of 5.4 × 106 in the 10-μm channel (Figure 13b). Meanwhile, a pristine rr-P3HT film generated charge carrier mobility, threshold voltage, and on/off ratio values of 0.10 cm2 V−1 s−1, −0.69 V, and 5.9 × 107, respectively (Figure 13c). OFET characteristics of the patterned rr-P3HT were almost the same as those of the pristine P3HT, indicating that the system was adaptable to the microfabrication of various other conductive polymers owing to its simple crosslinking mechanism.
Plots of Ids and Ids1/2 versus Vgs using (a) devices with patterned rr-P3HT, (b) patterned and dedoped rr-P3HT, and (c) pristine rr-P3HT (L=10 μm; W=500 μm). Reproduced with modifications with permission from ref. 96. Copyright 2011 Royal Society of Chemistry.
Summary and outlook
In this review, recent progress in thermally stable and photosensitive polymers (TSPSPs) during the last decade was introduced, with a focus on TSPSP design and application. Significant advancements have been achieved in the development of PSPIs, PSPBOs, and photosensitive engineering plastics. The three main requirements of PSPIs and PSPBOs include the following: (i) direct formulation from PAA or PHA, (ii) high sensitivity, and (iii) low-temperature cyclization. A simple formulation of TSPSPs has been achieved through direct mixing of precursors of PI or PBO with additives such as DNQ, PAG, and PBG or through mixing of engineering plastics with a crosslinker and PAG. Introduction of the CA mechanism into TSPSPs has also significantly improved their photosensitivity. PAG and PBG promote low-temperature cyclization of PAA and PHA. Applications for TSPSPs are expanding, and property requirements are continually being made stricter. For example, the biocompatibility of PSPIs for implantable medical device use has been studied,97 and the following conclusions have been drawn: (i) PSPI is noncytotoxic compared with the negative control of polyethylene and to the conventional PIs tested; (ii) fibroblast adhesion, morphology, and spreading levels are better when using a PSPI substrate than when using PIs. Accordingly, PSPIs can be used for biological microsystem and neuroprosthetic applications. Meanwhile, next-generation TSPSPs for electronic packaging should satisfy conflicting properties (for example, high modulus and elongation levels or low modulus and CTE levels).98, 99 Therefore, basic and applied research studies of TSPSPs should continue to reveal fundamental information on their molecular design and basic properties as well as to attain the required properties described above.

Transformation of diazonaphthoquinones (DNQs) derivatives upon UV exposure. Reproduced with modifications with permission from ref. 10. Copyright 1983 American Chemical Society.

Chemical amplification (CA) system of the poly(4-t-butoxycarbonyloxystyrene) resist.11

Photochemical generation of an alicyclic secondary amine. Reproduced with modifications with permission from ref. 17. Copyright 2006 Wiley Periodicals.

Patterning process of a photosensitive polyhydroxyimide (PHI). Reproduced with modifications with permission from ref. 24. Copyright 2009 Wiley Periodicals.

Synthesis of a partially esterified poly(amic acid) (PAA).25

Patterning process of a low-temperature curable photosensitive polyimide (PSPI). Reproduced with modifications with permission from ref. 26. Copyright 2009 Wiley Periodicals.

Patterning process of a photosensitive fluorinated PAA (FPAA). Reproduced with modifications with permission from ref. 28. Copyright 2010 The Society of Photopolymer Science and Technology (SPST).

Patterning process of photosensitive fluorinated PAA (FPAA) and fluorinated diazonaphthoquinone (FDNQ) systems. Reproduced with modifications with permission from ref. 29. Copyright 2013 The Society of Photopolymer Science and Technology (SPST).

Patterning process of a two-layered system. Reproduced with modifications with permission from ref. 30. Copyright 2010 Elsevier.

Patterning process of a resist based on fluorinated PAA (FPAA), poly(amic acid) (PAA), and fluorinated diazonaphthoquinone (FDNQ). Reproduced with modifications with permission from ref. 31. Copyright 2013 The Royal Society of Chemistry.

Synthesis of polyimides (PIs)-containing siloxane and hydroxyl imide units.33

Patterning process of low CTE photosensitive polyimide (PSPI). Reproduced with modifications with permission from ref. 37. Copyright 2010 Wiley Periodicals.

The patterning process of a polybenzoxazole (PSPBO) system using a photobase generator (PBG) and an active ester-type crosslinker. Reproduced with modifications with permission from ref. 39. Copyright 2009 American Chemical Society.

Patterning process using the positive-type polybenzoxazole (PSPBO) based on poly(hydroxyl amide) (PHA), t-Boc DI, and (5-propylsulfonyloxyimino-5H-thiophen-2-ylidene)-(2-methylphenyl)acetonitrile (PTMA). Reproduced with modifications with permission from ref. 49. Copyright 2007 Wiley Periodicals.

Patterning process and subsequent low-temperature cyclization using positive-type polybenzoxazole (PSPBO). Reproduced with modifications with permission from ref. 56. Copyright 2007 The Society of Polymer Science, Japan.

Patterning process using negative-type photosensitive poly(ether ether sulfone) (PSPEES). Reproduced with modifications with permission from ref. 57. Copyright 2008 The Society of Polymer Science, Japan.

Mechanism of reaction-development patterning (RDP).59

Mechanism of chemical amplification with an acid amplifier for reaction development patterning.63

Preparation of PSPI-TiO2 nanocomposite. Reproduced with modifications with permission from ref. 75. Copyright 2008 American Chemical Society.
References
Omote, T. in Polyimides Fundamentals and Applications (eds Ghosh, M. K. & Mittal, K. L.) Ch. 5, 121–149 (Marcel Dekker, Inc., New York, NY, USA, 1996)
Asano, M. & Hiramoto, H. in Photosensitive Polyimides: Fundamentals and Applications (eds Horie, K. & Yamashita, T.) Ch. 5, 121–152 (Technomic, Lancaster, PA, USA, 1995)
Yoda, N. Recent development of advanced functional polymers for semiconductor encapsulants of integrated circuit chips and high-temperature photoresist for electronic applications. Polym. Adv. Technol. 8, 215–226 (1997).
Rubner, R. Innovation via photosensitive polyimide and poly (benzoxazole) precursors—a review by inventor. J. Photopolym. Sci. Technol. 17, 685–691 (2004).
Mochizuki, A. & Ueda, M. Recent development in photosensitive polyimide (PSPI). J. Photopolym. Sci. Technol. 14, 677–687 (2001).
Ahne, H., Leuschner, R. & Rubner, R. Recent advances in photosensitive polyimides. Polym. Adv. Technol. 4, 217–233 (1993).
Fukukawa, K. I. & Ueda, M. Recent development of photosensitive polybenzoxazoles. Polym. J. 38, 405–418 (2006).
Fukukawa, K.-I. & Ueda, M. Developments of photosensitive polyimides and photosensitive polybenzoxazoles. Kobunshi Ronbunshu 63, 561–576 (2006).
Fukukawa, K.-i. & Ueda, M. Recent progress of photosensitive polyimides. Polym. J. 40, 281 (2008).
Willson, C. G. in Introduction to microlithography Vol. 219 (eds Thompson, L. F., Willson, C. G. & Bowden, M. J.) Ch. 3, 87–159 (ACS, Washington, DC, USA, 1983)
Ito, H. Chemical amplification resists for microlithography. Adv. Polym. Sci. 172, 37–245 (2005).
Sakuma, T., Ogitani, S. & Ikeda, A. Study on thermal curing in ester type photosensitive polyimide. J. Photopolym. Sci. Technol. 8, 277–280 (1995).
Li, W., Shen, Z., Zheng, J. & Tang, S. Ft-ir study of the imidization process of photosensitive polyimide PMDA/ODA. Appl. Spectrosc. 52, 985–989 (1998).
Shin, T. J. & Ree, M. In situ infrared spectroscopy study on imidization reaction and imidization-induced refractive index and thickness variations in microscale thin films of a poly (amic ester). Langmuir 21, 6081–6085 (2005).
Unsal, E. & Cakmak, M. Real-time characterization of physical changes in polyimide film formation: From casting to imidization. Macromolecules 46, 8616–8627 (2013).
Windrich, F., Kappert, E. J., Malanin, M., Eichhorn, K.-J., Häuβler, L., Benes, N. E. & Voit, B. In-situ imidization analysis in microscale thin films of an ester-type photosensitive polyimide for microelectronic packaging applications. Eur. Polym. J. 84, 279–291 (2016).
Fukukawa, K. I., Shibasaki, Y. & Ueda, M. Direct patterning of poly (amic acid) and low-temperature imidization using a photo-base generator. Polym. Adv. Technol. 17, 131–136 (2006).
Fukuda, S., Katayama, M. & Sakayori, K. Photosensitive polyimide using a highly sensitive photobase generator. J. Photopolym. Sci. Technol. 22, 391–392 (2009).
Fukuda, S., Amagai, K., Kanke, S. & Sakayori, K. Photosensitive polyimide using a highly sensitive photobase generator (2). J. Photopolym. Sci. Technol. 24, 267–268 (2011).
Ueda, M. & Nakayama, T. A new negative-type photosensitive polyimide based on poly (hydroxyimide), a cross-linker, and a photoacid generator. Macromolecules 29, 6427–6431 (1996).
Omote, T., Koseki, K. I. & Yamaoka, T. Fluorine-containing photoreactive polyimide. 6. Synthesis and properties of a novel photoreactive polyimide based on photo-induced acidolysis and the kinetics for its acidolysis. Macromolecules 23, 4788–4795 (1990).
Jin, X. Z. & Ishii, H. Novel positive-type photosensitive polyimide with low dielectric constant. J. Appl. Polym. Sci. 98, 15–21 (2005).
Jung, M. S., Hyeon-Lee, J. & Choi, T. L. A positive-working photosensitive polyimide based on thermal cross-linking and acidolytic cleavage. J. Appl. Polym. Sci. 107, 2632–2637 (2008).
Saito, Y., Mizoguchi, K., Higashihara, T. & Ueda, M. Alkaline-developable, chemically amplified, negative-type photosensitive polyimide based on polyhydroxyimide, a crosslinker, and a photoacid generator. J. Appl. Polym. Sci. 113, 3605–3611 (2009).
Tomikawa, M., Yoshida, S. & Okamoto, N. Novel partial esterification reaction in poly (amic acid) and its application for positive-tone photosensitive polyimide precursor. Polym. J. 41, 604–608 (2009).
Ogura, T., Higashihara, T. & Ueda, M. Direct patterning of poly (amic acid) and low-temperature imidization using a crosslinker, a photoacid generator, and a thermobase generator. J. Polym. Sci. A: Polym. Chem. 47, 3362–3369 (2009).
Haba, O., Okazaki, M., Nakayama, T. & Ueda, M. Positive-working alkaline-developable photosensitive polyimide precursor based on poly (amic acid) and dissolution inhibitor. J. Photopolym. Sci. Technol. 10, 55–60 (1997).
Sugiyama, M., Ogura, T., Higashihara, T. & Ueda, M. Development of a chemically amplified photosensitive polyimide based on poly (amic acid), a dissolution inhibitor, and a photoacid generator. J. Photopolym. Sci. Technol. 23, 483–488 (2010).
Inoue, Y., Higashihara, T. & Ueda, M. Alkaline-developable positive-type photosensitive polyimide based on fluorinated poly (amic acid) and fluorinated diazonaphthoquinone. J. Photopolym. Sci. Technol. 26, 351–356 (2013).
Ogura, T., Higashihara, T. & Ueda, M. Pattern formation of polyimide by using photosensitive polybenzoxazole as a top layer. Eur. Polym. J. 46, 1576–1581 (2010).
Inoue, Y., Saito, Y., Higashihara, T. & Ueda, M. Facile formulation of alkaline-developable positive-type photosensitive polyimide based on fluorinated poly (amic acid), poly (amic acid), and fluorinated diazonaphthoquinone. J. Mater. Chem. C 1, 2553–2560 (2013).
Yuba, T., Okuda, R., Tomikawa, M. & Kim, J. H. Soft baking effect on lithographic performance by positive tone photosensitive polyimide. J. Photopolym. Sci. Technol. 23, 775–779 (2010).
Ishi, J., Sunaga, T., Nomura, M. & Kanaya, H. Organo-soluble polyimides and their applications to photosensitive cover layer materials in flexible printed circuit boards. J. Photopolym. Sci. Technol. 21, 107–112 (2008).
Ishii, J., Kanaya, H., Sunaga, T., Iwata, M. & Nomura, M. Ultra-low-modulus positive-type photosensitive polyimides—improvement of adhesion strength with copper foil. J. Adhes. Soc. Jpn. 46, 137–144 (2010).
Hasegawa, M. Semi-aromatic polyimides with low dielectric constant and low cte. High Perform. Polym. 13, 93–106 (2001).
Hasegawa, M. & Koyanaka, M. Polyimides containing trans-1, 4-cyclohexane unit. Polymerizability of their precursors and low-cte, low-k and high-tg properties. High Perform. Polym. 15, 47–64 (2003).
Ogura, T., Higashihara, T. & Ueda, M. Low-CTE photosensitive polyimide based on semialicyclic poly (amic acid) and photobase generator. J. Polym. Sci. A: Polym. Chem. 48, 1317–1323 (2010).
Ogura, T., Saito, Y., Higashihara, T. & Ueda, M. Formation of spherical nanoparticles in poly (amic acid) films. Polym. Chem. 3, 2165–2169 (2012).
Mizoguchi, K., Higashihara, T. & Ueda, M. An alkaline-developable, chemically amplified, negative-type photosensitive poly (benzoxazole) resist based on poly (o-hydroxy amide), an active ester-type cross-linker, and a photobase generator. Macromolecules 42, 1024–1030 (2009).
Mizoguchi, K., Higashihara, T. & Ueda, M. An alkaline-developable, negative-working photosensitive polybenzoxazole based on poly (o-hydroxyamide), a vinyl sulfone-type cross-linker, and a novel photobase generator. Macromolecules 42, 3780–3787 (2009).
Wu, X. & Cooperman, B. S. Synthesis and biological activity of a bivalent nucleotide inhibitor of ribonucleotide reductase. Bioorg. Med. Chem. Lett. 10, 2387–2389 (2000).
Martina, S., MacDonald, S. A. & Enkelmann, V. Photosensitive tetramethylpiperidine urethanes: synthesis and characterization. J. Org. Chem. 59, 3281–3283 (1994).
Pirrung, M. C., Lee, Y. R., Park, K. & Springer, J. B. Pentadienylnitrobenzyl and pentadienylnitropiperonyl photochemically removable protecting groups. J. Org. Chem. 64, 5042–5047 (1999).
Rubner, R. Photoreactive polymers for electronics. Adv. Mater. 2, 452–457 (1990).
Yamaoka, T., Nakajima, N., Koseki, K. I. & Maruyama, Y. A study of novel heat-resistant polymers: preparation of photosensitive fluorinated polybenzoxazole precursors and physical properties of polybenzoxazoles derived from the precursors. J. Polym. Sci. A: Polym. Chem. 28, 2517–2532 (1990).
Ebara, K., Shibasaki, Y. & Ueda, M. Photosensitive poly (benzoxazole) based on precursor from diphenyl isophthalate and bis (o-aminophenol). Polymer 44, 333–339 (2003).
Ebara, K., Shibasaki, Y. & Ueda, M. New synthetic route for photosensitive poly (benzoxazole). J. Polym. Sci. A: Polym. Chem. 40, 3399–3405 (2002).
Fukukawa, K., Shibasaki, Y. & Ueda, M. Photosensitive poly(benzoxazole) via poly(o-hydroxy azomethine) ii. Environmentally benign process in ethyl lactate. Polym. J. 36, 489–494 (2004).
Ogura, T. & Ueda, M. Photosensitive polybenzoxazole based on a poly (o-hydroxy amide), a dissolution inhibitor, and a photoacid generator. J. Polym. Sci. A: Polym. Chem 45, 661–668 (2007).
Ando, S., Fujigaya, T. & Ueda, M. Density functional theory calculations of photoabsorption spectra of organic molecules in the vacuum ultraviolet region. Jpn. J. Appl. Phys. 41, 105–108 (2002).
Toyokawa, F., Fukukawa, K. I., Shibasaki, Y., Ando, S. & Ueda, M. Synthesis of a highly transparent poly (o-hydroxyamide) in the i-line region and its application to photosensitive polymers. J. Polym. Sci. A: Polym. Chem. 43, 2527–2535 (2005).
Shibasaki, Y., Toyokawa, F., Ando, S. & Ueda, M. Highly transparent photosensitive polybenzoxazole: poly (o-hydroxy amide) derived from 4, 4′-(hexafluoroisopropylidene) bis (o-aminophenol) and o-substituted dicarboxylic acid chlorides. Polym. J. 39, 81–89 (2007).
Toyokawa, F., Fukukawa, K.-i., Shibasaki, Y. & Ueda, M. An efficient catalyst for low temperature solid-phase cyclization of poly (o-hydroxyamide). Chem. Lett. 33, 1342–1343 (2004).
Toyokawa, F., Shibasaki, Y. & Ueda, M. A novel low temperature curable photosensitive polybenzoxazole. Polym. J. 37, 517–521 (2005).
Fukukawa, K.-i. & Ueda, M. Facile synthesis of photosensitive poly (semi-alicyclic benzoxazole) and subsequent low-temperature cyclization of poly (o-hydroxy amide). Macromolecules 39, 2100–2106 (2006).
Ogura, T., Yamaguchi, K.-T., Shibasaki, Y. & Ueda, M. Photosensitive poly (benzoxazole) based on poly (o-hydroxy amide), dissolution inhibitor, thermoacid generator, and photoacid generator. Polym. J. 39, 245–251 (2007).
Mizoguchi, K. & Ueda, M. Direct patterning of poly (ether ether sulfone) using a cross-linker and a photoacid generator. Polym. J. 40, 645–650 (2008).
Saito, Y., Matsumoto, K., Higashihara, T. & Ueda, M. A chemically amplified, negative-type photosensitive poly (phenylene ether ketone)(PEK) resist based on ketal-protected pek and a photoacid generator. Chem. Lett. 38, 1048–1049 (2009).
Fukushima, T., Oyama, T., Iijima, T., Tomoi, M. & Itatani, H. New concept of positive photosensitive polyimide: reaction development patterning (RDP). J. Polym. Sci. A: Polym. Chem. 39, 3451–3463 (2001).
Sugawara, S., Tomoi, M. & Oyama, T. Photosensitive polyesterimides based on reaction development patterning. Polym. J. 39, 129–137 (2007).
Kawada, T., Takahashi, A. & Oyama, T. Addition of photosensitivity to hyperbranched engineering plastics based on reaction development patterning. J. Photopolym. Sci. Technol. 27, 219–222 (2014).
Yasuda, M., Takahashi, A. & Oyama, T. Development of photosensitive alicyclic polyimides based on reaction development patterning. J. Photopolym. Sci. Technol. 26, 357–360 (2013).
Cheng, X., Takahashi, A. & Oyama, T. Development of chemically amplified reaction development patterning. Polym. J. 42, 86–94 (2010).
Regolini, J., Benoit, D. & Morin, P. Passivation issues in active pixel cmos image sensors. Microelectron. Reliabil. 47, 739–742 (2007).
Liu, J.-g. & Ueda, M. High refractive index polymers: fundamental research and practical applications. J. Mater. Chem. 19, 8907–8919 (2009).
Higashihara, T. & Ueda, M. Recent progress in high refractive index polymers. Macromolecules 48, 1915–1929 (2015).
Liu, J.-g., Nakamura, Y., Shibasaki, Y., Ando, S. & Ueda, M. Synthesis and characterization of high refractive index polyimides derived from 4,4′-(p-phenylenedisulfanyl)dianiline and various aromatic tetracarboxylic dianhydrides. Polym. J. 39, 543–550 (2007).
Liu, J.-g., Nakamura, Y., Shibasaki, Y., Ando, S. & Ueda, M. High refractive index polyimides derived from 2, 7-bis (4-aminophenylenesulfanyl) thianthrene and aromatic dianhydrides. Macromolecules 40, 4614–4620 (2007).
Liu, J. G., Nakamura, Y., Terraza, C. A., Shibasaki, Y., Ando, S. & Ueda, M. Highly refractive polyimides derived from 2, 8-bis (p-aminophenylenesulfanyl) dibenzothiophene and aromatic dianhydrides. Macromol. Chem. Phys. 209, 195–203 (2008).
You, N.-H., Suzuki, Y., Yorifuji, D., Ando, S. & Ueda, M. Synthesis of high refractive index polyimides derived from 1, 6-bis (p-aminophenylsulfanyl)-3, 4, 8, 9-tetrahydro-2, 5, 7, 10-tetrathiaanthracene and aromatic dianhydrides. Macromolecules 41, 6361–6366 (2008).
You, N.-H., Suzuki, Y., Higashihara, T., Ando, S. & Ueda, M. Synthesis and characterization of highly refractive polyimides derived from 2, 7-bis (4′-aminophenylenesulfanyl) thianthrene-5, 5, 10, 10-tetraoxide and aromatic dianhydrides. Polymer 50, 789–795 (2009).
Fukuzaki, N., Higashihara, T., Ando, S. & Ueda, M. Synthesis and characterization of highly refractive polyimides derived from thiophene-containing aromatic diamines and aromatic dianhydrides. Macromolecules 43, 1836–1843 (2010).
You, N.-H., Higashihara, T., Oishi, Y., Ando, S. & Ueda, M. Highly refractive poly (phenylene thioether) containing triazine unit. Macromolecules 43, 4613–4615 (2010).
Nakabayashi, K., Imai, T., Fu, M.-C., Ando, S., Higashihara, T. & Ueda, M. Poly (phenylene thioether) s with fluorene-based cardo structure toward high transparency, high refractive index, and low birefringence. Macromolecules 49, 5849–5856 (2016).
Liu, J.-g., Nakamura, Y., Ogura, T., Shibasaki, Y., Ando, S. & Ueda, M. Optically transparent sulfur-containing polyimide−TiO2 nanocomposite films with high refractive index and negative pattern formation from poly(amic acid)−TiO2 nanocomposite film. Chem. Mater. 20, 273–281 (2007).
Tomikawa, M., Suwa, M., Niwa, H. & Minamihashi, K. Novel high refractive index positive-tone photosensitive polyimide for microlens of image sensors. High Perform. Polym. 23, 66–73 (2011).
Saito, Y., Higashihara, T. & Ueda, M. Highly refractive and photosensitive polyimide. J. Photopolym. Sci. Technol. 22, 423–428 (2009).
Ogura, T., Higashihara, T. & Ueda, M. Development of photosensitive poly (hydroxyimide) with high refractive index. J. Photopolym. Sci. Technol. 23, 515–520 (2010).
Tsuchiya, K., Shibasaki, Y. & Ueda, M. A negative type photosensitive polymer based on poly (naphthylene ether), a cross-linker, and a photoacid generator with low dielectric constant. Polym. J. 39, 442–447 (2007).
Long, T. M. & Swager, T. M. Molecular design of free volume as a route to low-κ dielectric materials. J. Am. Chem. Soc. 125, 14113–14119 (2003).
Mizoguchi, K. & Ueda, M. Negative-type photosensitive poly (phenylene ether) based on poly (2, 6-dimethyl-1, 4-phenylene ether), a crosslinker, and a photoacid generator. J. Polym. Sci. A: Polym. Chem. 46, 4949–4958 (2008).
Mizoguchi, K., Higashihara, T. & Ueda, M. Negative-working photosensitive poly (phenylene ether) based on poly (2,6-dimethyl-1, 4-phenylene ether), a cross-linker, and a photoacid generator. Macromolecules 43, 2832–2839 (2010).
Berry, A. K., Graziano, K. A., Bogan, L. E. Jr & Thackeray, J. W. in Polymers in Microlithography Materials and Processes Vol. 412 (eds Reichmanis, E., MacDonald, S. A. & Iwayanagi, T.) Ch. 6, 86–99 (ACS, Washington, DC, USA, 1989)
Saito, Y., Mizoguchi, K. & Ueda, M. Negative-type chemically amplified photosensitive novolac. J. Photopolym. Sci. Technol. 21, 161–164 (2008).
Coakley, K. M. & McGehee, M. D. Conjugated polymer photovoltaic cells. Chem. Mater. 16, 4533–4542 (2004).
Günes, S., Neugebauer, H. & Sariciftci, N. S. Conjugated polymer-based organic solar cells. Chem. Rev. 107, 1324–1338 (2007).
Naber, R., Mulder, M., De Boer, B., Blom, P. & De Leeuw, D. High charge density and mobility in poly (3-hexylthiophene) using a polarizable gate dielectric. Org. Electron. 7, 132–136 (2006).
Manceau, M., Rivaton, A. & Gardette, J. L. Involvement of singlet oxygen in the solid-state photochemistry of P3HT. Macromol. Rapid Commun. 29, 1823–1827 (2008).
Holdcroft, S. Patterning pi-conjugated polymers. Adv. Mater. 13, 1753–1765 (2001).
Park, S. K., Kim, Y. H., Han, J. I., Moon, D. G., Kim, W. K. & Kwak, M. G. Electrical characteristics of poly (3-hexylthiophene) thin film transistors printed and spin-coated on plastic substrates. Synth. Met. 139, 377–384 (2003).
Abdou, M. S., Diaz-Guijada, G. A., Arroyo, M. I. & Holdcroft, S. Photoimaging of electronically conducting polymeric networks. Chem. Mater. 3, 1003–1006 (1991).
Chang, J. F. & Sirringhaus, H. Patterning of solution-processed semiconducting polymers in high-mobility thin-film transistors by physical delamination. Adv. Mater. 21, 2530–2535 (2009).
Qiu, L., Xu, Q., Lee, W. H., Wang, X., Kang, B., Lv, G. & Cho, K. Organic thin-film transistors with a photo-patternable semiconducting polymer blend. J. Mater. Chem. 21, 15637–15642 (2011).
Endo, K., Ogura, T., Higashihara, T. & Ueda, M. A negative-type photosensitive poly (3-hexylthiophene) with cross-linker and photoacid generator. Polym. J. 41, 808–809 (2009).
Saito, Y., Higashihara, T. & Ueda, M. Direct photo-patterning of poly (3-hexylthiophene). J. Photopolym. Sci. Technol. 24, 273–276 (2011).
Saito, Y., Sakai, Y., Higashihara, T. & Ueda, M. Direct patterning of poly (3-hexylthiophene) and its application to organic field-effect transistor. RSC Adv. 2, 1285–1288 (2012).
Sun, Y., Lacour, S., Brooks, R., Rushton, N., Fawcett, J. & Cameron, R. Assessment of the biocompatibility of photosensitive polyimide for implantable medical device use. J. Biomed. Mater. Res. A 90, 648–655 (2009).
Shoji, Y., Koyama, Y., Masuda, Y., Hashimoto, K., Isobe, K. & Okuda, R. Development of novel low-temperature curable positive-tone photosensitive polyimide with high elongation. J. Photopolym. Sci. Technol. 29, 277–282 (2016).
Tomikawa, M., Okuda, R. & Ohnishi, H. Photosensitive polyimide for packaging applications. J. Photopolym. Sci. Technol. 28, 73–77 (2015).
Acknowledgements
M-CF acknowledges Yamagata University’s Innovative Flex Course for Frontier Organic Material Systems (iFront) for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fu, MC., Higashihara, T. & Ueda, M. Recent progress in thermally stable and photosensitive polymers. Polym J 50, 57–76 (2018). https://doi.org/10.1038/pj.2017.46
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.46