Abstract
Background:
Prostate cancer remains a significant health problem for men in the Western world. Although treatment modalities are available, these do not confer long-term benefit and are accompanied by substantial side effects. Adoptive immunotherapy represents an attractive alternative to conventional treatments as a means to control tumor growth.
Methods:
To selectively target the tumor-expressed form of Muc1 we constructed a retroviral vector encoding a chimeric antigen receptor (CAR) directed against the aberrantly-expressed extracellular portion of Muc1 called the ‘variable number of tandem repeats’.
Results:
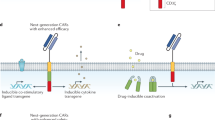
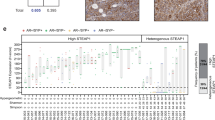
We now demonstrate that T cells can be genetically engineered to express a CAR targeting the tumor-associated antigen Muc1. CAR-Muc1 T cells were able to selectively kill Muc1-expressing human prostate cancer cells. However, we noted that heterogeneous expression of the Muc1 antigen on tumor cells facilitated immune escape and the outgrowth of target-antigen loss variants of the tumor. Given the importance of androgen ablation therapy in the management of metastatic prostate cancer, we therefore also tested the value of combining conventional (anti-androgen) and experimental (CAR-Muc1 T cells) approaches. We show that CAR-Muc1 T cells were not adversely impacted by anti-androgen therapy and subsequently demonstrate the feasibility of combining the approaches to produce additive anti-tumor effects in vitro.
Conclusions:
Adoptive transfer of CAR-Muc1 T cells alone or in combination with other luteinizing hormone-releasing hormone analogs or antagonists should be tested in human clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422.
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24: 3089–3094.
Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009; 115: 3670–3679.
Karan D, Holzbeierlein JM, Van Veldhuizen P, Thrasher JB . Cancer immunotherapy: a paradigm shift for prostate cancer treatment. Nat Rev Urol 2012; 9: 376–385.
Longo DL . New therapies for castration-resistant prostate cancer. N Engl J Med 2010; 363: 479–481.
Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008; 14: 1264–1270.
Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011; 118: 6050–6056.
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011; 121: 1822–1826.
Eshhar Z, Waks T, Gross G, Schindler DG . Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA 1993; 90: 720–724.
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15: 5323–5337.
Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ et al. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis 2005; 22: 565–573.
Li Y, Cozzi PJ . MUC1 is a promising therapeutic target for prostate cancer therapy. Curr Cancer Drug Targets 2007; 7: 259–271.
Li Y, Cozzi PJ, Russell PJ . Promising tumor-associated antigens for future prostate cancer therapy. Med Res Rev 2010; 30: 67–101.
Beatson RE, Taylor-Papadimitriou J, Burchell JM . MUC1 immunotherapy. Immunotherapy 2010; 2: 305–327.
Kufe DW . Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther 2009; 8: 1197–1203.
Maher J, Wilkie S . CAR mechanics: driving T cells into the MUC of cancer. Cancer Res 2009; 69: 4559–4562.
Yonezawa S, Goto M, Yamada N, Higashi M, Nomoto M . Expression profiles of MUC1, MUC2, and MUC4 mucins in human neoplasms and their relationship with biological behavior. Proteomics 2008; 8: 3329–3341.
Singh R, Bandyopadhyay D . MUC1: a target molecule for cancer therapy. Cancer Biol Ther 2007; 6: 481–486.
Lapointe J, Li C, Higgins JP, van de Rijn M, Bair E, Montgomery K et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA 2004; 101: 811–816.
Engelmann K, Baldus SE, Hanisch FG . Identification and topology of variant sequences within individual repeat domains of the human epithelial tumor mucin MUC1. J Biol Chem 2001; 276: 27764–27769.
Baldus SE, Engelmann K, Hanisch FG . MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci 2004; 41: 189–231.
Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol 2008; 180: 4901–4909.
Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009; 360: 2516–2527.
Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 2011; 365: 107–118.
Antonarakis ES, Drake CG . Combining immunological and androgen-directed approaches: an emerging concept in prostate cancer immunotherapy. Curr Opin Oncol 2012; 24: 258–265.
Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006; 108: 3890–3897.
Hanisch FG, Muller S . MUC1: the polymorphic appearance of a human mucin. Glycobiology 2000; 10: 439–449.
Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK . A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 2005; 12: 933–941.
Tevell A, Lennernäs H, Jönsson M, Norlin M, Lennernäs B, Bondesson U et al. Flutamide metabolism in four different species in vitro and identification of flutamide metabolites in human patient urine by high performance liquid chromatography/tandem mass spectrometry. Drug Metab Dispos 2006; 34: 984–992.
Belanger A, Giasson M, Couture J, Dupont A, Cusan L, Labrie F . Plasma levels of hydroxy-flutamide in patients with prostatic cancer receiving the combined hormonal therapy: an LHRH agonist and flutamide. Prostate 1988; 12: 79–84.
Mosca A, Berruti A, Russo L, Torta M, Dogliotti L . The neuroendocrine phenotype in prostate cancer: basic and clinical aspects. J Endocrinol Invest 2005; 28: 141–145.
Zellweger T, Ninck C, Bloch M, Mirlacher M, Koivisto PA, Helin HJ et al. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer 2005; 113: 619–628.
Frank MO, Kaufman J, Tian S, Suárez-Fariñas M, Parveen S, Blachère NE et al. Harnessing naturally occurring tumor immunity: a clinical vaccine trial in prostate cancer. PLoS One 2010; 5: e12367.
Noguchi M, Uemura H, Naito S, Akaza H, Yamada A, Itoh K . A phase I study of personalized peptide vaccination using 14 kinds of vaccine in combination with low-dose estramustine in HLA-A24-positive patients with castration-resistant prostate cancer. Prostate 2011; 71: 470–479.
Harrop R, Shingler W, Kelleher M, de Belin J, Treasure P . Cross-trial analysis of immunologic and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J Immunother 2010; 33: 999–1005.
Weber JS, Vogelzang NJ, Ernstoff MS, Goodman OB, Cranmer LD, Marshall JL et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J Immunother 2011; 34: 556–567.
Chudley L, McCann K, Mander A, Tjelle T, Campos-Perez J, Godeseth R et al. DNA fusion-gene vaccination in patients with prostate cancer induces high-frequency CD8(+) T-cell responses and increases PSA doubling time. Cancer Immunol Immunother 2012; 61: 2161–2170.
Vieweg J, Dannull J . Technology Insight: vaccine therapy for prostate cancer. Nat Clin Pract Urol 2005; 2: 44–51.
Leen AM, Rooney CM, Foster AE . Improving T cell therapy for cancer. Annu Rev Immunol 2007; 25: 243–265.
Porter DL, Levine BL, Kalos M, Bagg A, June CH . Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733.
Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011; 3: 95ra73.
Vera JF, Brenner MK, Dotti G . Immunotherapy of human cancers using gene modified T lymphocytes. Curr Gene Ther 2009; 9: 396–408.
Ebelt K, Babaryka G, Frankenberger B, Stief CG, Eisenmenger W, Kirchner T et al. Prostate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clusters. Eur J Cancer 2009; 45: 1664–1672.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF et al. Safety, activity, and immune correlates of anti-pd-1 antibody in cancer. N Engl J Med 2012; 366: 2443–2454.
Durrett R, Foo J, Leder K, Mayberry J, Michor F . Evolutionary dynamics of tumor progression with random fitness values. Theor Popul Biol 2010; 78: 54–66.
Durrett R, Foo J, Leder K, Mayberry J, Michor F . Intratumor heterogeneity in evolutionary models of tumor progression. Genetics 2011; 188: 461–477.
Kim W, Ryan CJ . Androgen receptor directed therapies in castration-resistant metastatic prostate cancer. Curr Treat Options Oncol 2012; 13: 189–200.
Mottet N, Van Damme J, Loulidi S, Russel C, Leitenberger A, Wolff JM . Intermittent hormonal therapy in the treatment of metastatic prostate cancer: a randomized trial. BJU Int 2012; 110: 1262–1269.
Dayyani F, Gallick GE, Logothetis CJ, Corn PG . Novel therapies for metastatic castrate-resistant prostate cancer. J Natl Cancer Inst 2011; 103: 1665–1675.
Reid AH, Attard G, Barrie E, de Bono JS . CYP17 inhibition as a hormonal strategy for prostate cancer. Nat Clin Pract Urol 2008; 5: 610–620.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324: 787–790.
Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 2010; 375: 1437–1446.
Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci USA 2001; 98: 14565–14570.
Morse MD, McNeel DG . T cells localized to the androgen-deprived prostate are T(H) 1 and T(H) 17 biased. Prostate 2011; 72: 1239–1247.
Morse MD, McNeel DG . Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol 2010; 71: 496–504.
Ellis TM, Moser MT, Le PT, Flanigan RC, Kwon ED . Alterations in peripheral B cells and B cell progenitors following androgen ablation in mice. Int Immunol 2001; 13: 553–558.
Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol 2005; 174: 539–546.
Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res 2008; 14: 4526–4531.
Acknowledgements
JFV is supported by an Idea Development Award from the Department of Defense Prostate Cancer Research Program (no. W81XWH-11-1-0625). The research work was also supported by the Adrienne Helis Malvin Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with Baylor College of Medicine. MKB is supported by a Fayez Sarofim Chair.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Sanchez, C., Chan, R., Bajgain, P. et al. Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer. Prostate Cancer Prostatic Dis 16, 123–131 (2013). https://doi.org/10.1038/pcan.2012.49
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2012.49
Keywords
This article is cited by
-
The potential of CAR T cell therapy for prostate cancer
Nature Reviews Urology (2021)
-
Management of men with metastatic castration-resistant prostate cancer following potent androgen receptor inhibition: a review of novel investigational therapies
Prostate Cancer and Prostatic Diseases (2021)
-
CAR-T cell therapy: a potential new strategy against prostate cancer
Journal for ImmunoTherapy of Cancer (2019)
-
Chimeric Antigen Receptor-Engineered T Cells for the Treatment of Metastatic Prostate Cancer
BioDrugs (2015)
-
Kinetics of Tumor Destruction by Chimeric Antigen Receptor-modified T Cells
Molecular Therapy (2014)