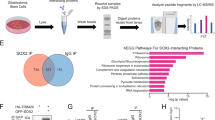
Abstract
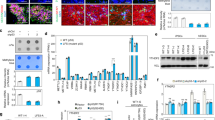
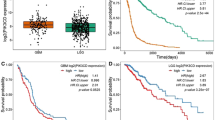
Wild-type p53 (wtp53) is described as a tumour suppressor gene; mutations in this gene occur in many human cancers and promote oncogenic capacity. Here, we establish that the oncogenic activity of mutant p53 (mtp53) is driven by the WASP-interacting protein (WIP). WIP knockdown from mtp53-expressing glioblastoma and breast cancer cells (BCC) greatly reduced proliferation and growth capacity of cancer stem cell (CSC)-like cells and decreased CSC-like markers (CD133, CD44 or YAP/TAZ). mtp53 overexpression in human astrocytes enhanced their proliferative capacity in suspension culture and increased expression of CSC markers and WIP. WIP knockdown compromised tumour glioblastoma and BCC growth capacity in vivo. We show that WIP is phosphorylated by AKT2 and is regulated by mtp53/p63 through enhancement of PI3K/AKT2-mediated integrin/receptor recycling pathways. WIP regulates this oncogenic pathway by controlling YAP/TAZ stability. We thus establish a new CSC signalling pathway downstream of mtp53 in which AKT2 regulates WIP and controls YAP/TAZ stability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Powell E, Piwnica-Worms D, Piwnica-Worms H . Contribution of p53 to metastasis. Cancer Discov 2014; 4: 405–414.
Suh YA, Post SM, Elizondo-Fraire AC, Maccio DR, Jackson JG, El-Naggar AK et al. Multiple stress signals activate mutant p53 in vivo. Cancer Res 2011; 71: 7168–7175.
Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell 2009; 137: 87–98.
Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 2009; 139: 1327–1341.
Weissmueller S, Manchado E, Saborowski M, JPt Morris, Wagenblast E, Davis CA et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell 2014; 157: 382–394.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Visvader JE, Lindeman GJ . Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8: 755–768.
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367: 645–648.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumourigenic breast cancer cells. PNAS 2003; 100: 3983–3988.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Nagpal J, Jamoona A, Gulati ND, Mohan A, Braun A, Murali R et al. Revisiting the role of p53 in primary and secondary glioblastomas. Anticancer Res 2006; 26: 4633–4639.
Shiraishi S, Tada K, Nakamura H, Makino K, Kochi M, Saya H et al. Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 2002; 95: 249–257.
Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009; 138: 645–659.
Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. PNAS 2011; 108: 16259–16264.
Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J et al. Molecular definition of breast tumour heterogeneity. Cancer Cell 2007; 11: 259–273.
Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011; 147: 759–772.
Piccolo S, Dupont S, Cordenonsi M . The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 2014; 94: 1287–1312.
Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol 2008; 10: 837–848.
Staub E, Groene J, Heinze M, Mennerich D, Roepcke S, Klaman I et al. An expression module of WIPF1-coexpressed genes identifies patients with favorable prognosis in three tumour types. J Mol Med 2009; 87: 633–644.
Anton IM, Jones GE, Wandosell F, Geha R, Ramesh N . WASP-interacting protein (WIP): working in polymerisation and much more. Trends Cell Biol 2007; 17: 555–562.
Banon-Rodriguez I, Saez de Guinoa J, Bernardini A, Ragazzini C, Fernandez E, Carrasco YR et al. WIP regulates persistence of cell migration and ruffle formation in both mesenchymal and amoeboid modes of motility. PloS One 2013; 8: e70364.
Lanzardo S, Curcio C, Forni G, Anton IM . A role for WASP interacting protein, WIP, in fibroblast adhesion, spreading and migration. Int J Biochem Cell Biol 2007; 39: 262–274.
Garcia E, Jones GE, Machesky LM, Anton IM . WIP: WASP-interacting proteins at invadopodia and podosomes. Eur J Cell Biol 2012; 91: 869–877.
Garcia E, Machesky LM, Jones GE, Anton IM . WIP is necessary for matrix invasion by breast cancer cells. Eur J Cell Biol 2014; 93: 413–423.
Muller PA, Vousden KH . p53 mutations in cancer. Nat Cell Biol 2013; 15: 2–8.
Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J et al. Growth-inhibitory and tumour- suppressive functions of p53 depend on its repression of CD44 expression. Cell 2008; 134: 62–73.
Mizuarai S, Yamanaka K, Kotani H . Mutant p53 induces the GEF-H1 oncogene, a guanine nucleotide exchange factor-H1 for RhoA, resulting in accelerated cell proliferation in tumour cells. Cancer Res 2006; 66: 6319–6326.
Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, Kao SH et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol 2009; 11: 694–704.
Rainero E, Caswell PT, Muller PA, Grindlay J, McCaffrey MW, Zhang Q et al. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J Cell Biol 2012; 196: 277–295.
Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer 2014; 14: 92–107.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–2761.
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT et al. Matrix crosslinking forces tumour progression by enhancing integrin signaling. Cell 2009; 139: 891–906.
Kim NG, Gumbiner BM . Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol 2015; 210: 503–515.
Fan R, Kim NG, Gumbiner BM . Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. PNAS 2013; 110: 2569–2574.
Klein C . Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu Rev Immunol 2011; 29: 399–413.
Anton IM, de la Fuente MA, Sims TN, Freeman S, Ramesh N, Hartwig JH et al. WIP deficiency reveals a differential role for WIP and the actin cytoskeleton in T and B cell activation. Immunity 2002; 16: 193–204.
Gallego MD, de la Fuente MA, Anton IM, Snapper S, Fuhlbrigge R, Geha RS . WIP and WASP play complementary roles in T cell homing and chemotaxis to SDF-1alpha. Int Immunol 2006; 18: 221–232.
Le Bras S, Massaad M, Koduru S, Kumar L, Oyoshi MK, Hartwig J et al. WIP is critical for T cell responsiveness to IL-2. PNAS 2009; 106: 7519–7524.
Franco A, Knafo S, Banon-Rodriguez I, Merino-Serrais P, Fernaud-Espinosa I, Nieto M et al. WIP is a negative regulator of neuronal maturation and synaptic activity. Cereb Cortex 2012; 22: 1191–1202.
Aspenstrom P . The verprolin family of proteins: regulators of cell morphogenesis and endocytosis. FEBS Lett 2005; 579: 5253–5259.
Thanabalu T, Rajmohan R, Meng L, Ren G, Vajjhala PR, Munn AL . Verprolin function in endocytosis and actin organization. Roles of the Las17p (yeast WASP)-binding domain and a novel C-terminal actin-binding domain. FEBS J 2007; 274: 4103–4125.
Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev 2011; 25: 2594–2609.
Hiemer SE, Varelas X . Stem cell regulation by the Hippo pathway. Biochim Biophys Acta 2013; 1830: 2323–2334.
Imajo M, Ebisuya M, Nishida E . Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 2015; 17: 7–19.
Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 2010; 24: 1106–1118.
Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Biol 2007; 17: 2054–2060.
Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 2014; 16: 357–366.
Yu FX, Guan KL . The Hippo pathway: regulators and regulations. Genes Dev 2013; 27: 355–371.
Piccolo S, Cordenonsi M, Dupont S . Molecular pathways: YAP and TAZ take center stage in organ growth and tumourigenesis. Clin Cancer Res 2013; 19: 4925–4930.
Zhao B, Lei QY, Guan KL . The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol 2008; 20: 638–646.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474: 179–183.
Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL . Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 2012; 26: 54–68.
Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F . Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 2011; 138: 2337–2346.
Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T et al. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumour suppressor. J Cell Biol 2000; 149: 1073–1086.
Lucas EP, Khanal I, Gaspar P, Fletcher GC, Polesello C, Tapon N et al. The Hippo pathway polarizes the actin cytoskeleton during collective migration of Drosophila border cells. J Cell Biol 2013; 201: 875–885.
Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J 2011; 30: 2325–2335.
Wada K, Itoga K, Okano T, Yonemura S, Sasaki H . Hippo pathway regulation by cell morphology and stress fibers. Development 2011; 138: 3907–3914.
Yang X, Yu K, Hao Y, Li DM, Stewart R, Insogna KL et al. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nat Cell Biol 2004; 6: 609–617.
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 2014; 158: 157–170.
Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S et al. Role of TAZ as mediator of Wnt signaling. Cell 2012; 151: 1443–1456.
Gargini R, Escoll M, García E, García-Escudero R, Wandosell F, Antón IM . WIP drives tumor progression through YAP/TAZ-dependent autonomous cell growth. Cell Rep 2016; 17: 1962–1977.
Gargini R, Cerliani JP, Escoll M, Anton IM, Wandosell F . Cancer stem cell-like phenotype and survival are coordinately regulated by Akt/FoxO/Bim pathway. Stem Cells 2015; 33: 646–660.
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D et al. Isolation and in vitro propagation of tumourigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005; 65: 5506–5511.
Rada P, Rojo AI, Chowdhry S, McMahon M, Hayes JD, Cuadrado A . SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 2011; 31: 1121–1133.
Werbowetski-Ogilvie TE, Agar NY, Waldkircher de Oliveira RM, Faury D, Antel JP, Jabado N et al. Isolation of a natural inhibitor of human malignant glial cell invasion: inter alpha-trypsin inhibitor heavy chain 2. Cancer Res 2006; 66: 1464–1472.
Acknowledgements
We thank Catherine Mark for editorial assistance. The proteomic analysis was performed in the proteomics facility of Centro Nacional de Biotecnología that belongs to ProteoRed, PRB2-ISCIII; we particularly acknowledge the exceptional work of Rosana Navajas. This work was supported by grants from the MICINN, the Plan Nacional of the Dirección General de Ciencia y Tecnología (SAF2012-39148-C03-01 to FW, SAF2013-45937-R to IMA and SAF2015-70368-R to both and SAF2013-43271-R to AC), the European Union (EU-FP7-2009-CT222887 to FW) and the Instituto de Salud Carlos III Centro de Investigación Biomédica en Red (CIBERNED).
Author contributions
ME and RG performed the experiments. ME, RG, IMA and FW designed the experiments and analysed the data, wrote the manuscript and provided editorial suggestions and criticisms. AC provided AKT-related reagents, suggestions and criticism.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Escoll, M., Gargini, R., Cuadrado, A. et al. Mutant p53 oncogenic functions in cancer stem cells are regulated by WIP through YAP/TAZ. Oncogene 36, 3515–3527 (2017). https://doi.org/10.1038/onc.2016.518
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.518
This article is cited by
-
Targeting ovarian cancer stem cells: a new way out
Stem Cell Research & Therapy (2023)
-
A meta-validated immune infiltration-related gene model predicts prognosis and immunotherapy sensitivity in HNSCC
BMC Cancer (2023)
-
Multidimensional quantitative phenotypic and molecular analysis reveals neomorphic behaviors of p53 missense mutants
npj Breast Cancer (2023)
-
Targeting p53 pathways: mechanisms, structures, and advances in therapy
Signal Transduction and Targeted Therapy (2023)
-
TP53 mutations upregulate RCP expression via Sp1/3 to drive lung cancer progression
Oncogene (2022)