Abstract
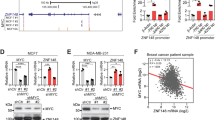
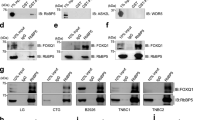
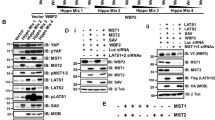
B-cell-specific Moloney murine leukemia virus integration site 1 (BMI1) is a component of the polycomb repressive complex 1 (PRC1) complex that is overexpressed in breast and other cancers, and promotes self-renewal of cancer stem-like cells. The oncogenic mucin 1 (MUC1) C-terminal (MUC1-C) subunit is similarly overexpressed in human carcinoma cells and has been linked to their self-renewal. There is no known relationship between MUC1-C and BMI1 in cancer. The present studies demonstrate that MUC1-C drives BMI1 transcription by a MYC-dependent mechanism in breast and other cancer cells. In addition, we show that MUC1-C blocks miR-200c-mediated downregulation of BMI1 expression. The functional significance of this MUC1-C→BMI1 pathway is supported by the demonstration that targeting MUC1-C suppresses BMI1-induced ubiquitylation of H2A and thereby derepresses homeobox HOXC5 and HOXC13 gene expression. Notably, our results further show that MUC1-C binds directly to BMI1 and promotes occupancy of BMI1 on the CDKN2A promoter. In concert with BMI1-induced repression of the p16INK4a tumor suppressor, we found that targeting MUC1-C is associated with induction of p16INK4a expression. In support of these results, analysis of three gene expresssion data sets demonstrated highly significant correlations between MUC1-C and BMI1 in breast cancers. These findings uncover a previously unrecognized role for MUC1-C in driving BMI1 expression and in directly interacting with this stem cell factor, linking MUC1-C with function of the PRC1 in epigenetic gene silencing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
Abbreviations
- BMI1:
-
B-cell-specific Moloney murine leukemia virus integration site 1
- PRC1:
-
polycomb repressive complex 1
- CSCs:
-
cancer stem-like cells
- EMT:
-
epithelial–mesenchymal transition
- DSB:
-
double-stranded DNA break
- MUC1:
-
mucin 1
- MUC1-C:
-
MUC1-C-terminal subunit
- DNMT:
-
DNA methyltransferase
- DOX:
-
doxycycline.
References
Park IK, Morrison SJ, Clarke MF . Bmi1, stem cells, and senescence regulation. J Clin Invest 2004; 113: 175–179.
Siddique HR, Saleem M . Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells 2012; 30: 372–378.
Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS et al. Role of histone H2A ubiquitination in Polycomb silencing. Nature 2004; 431: 873–878.
de Napoles M, Mermoud JE, Wakao R, Tang YA, Endoh M, Appanah R et al. Polycomb group proteins Ring1A/B link ubiquitylation of histone H2A to heritable gene silencing and X inactivation. Dev Cell 2004; 7: 663–676.
Cao R, Tsukada Y, Zhang Y . Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell 2005; 20: 845–854.
Ohtsubo M, Yasunaga S, Ohno Y, Tsumura M, Okada S, Ishikawa N et al. Polycomb-group complex 1 acts as an E3 ubiquitin ligase for Geminin to sustain hematopoietic stem cell activity. Proc Natl Acad Sci USA 2008; 105: 10396–10401.
Wang Y, Zhe H, Ding Z, Gao P, Zhang N, Li G . Cancer stem cell marker Bmi-1 expression is associated with basal-like phenotype and poor survival in breast cancer. World J Surg 2012; 36: 1189–1194.
Crea F, Paolicchi E, Marquez VE, Danesi R . Polycomb genes and cancer: time for clinical application? Crit Rev Oncol Hematol 2012; 83: 184–193.
Richly H, Aloia L, Di Croce L . Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis 2011; 2: e204.
Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med 2014; 20: 29–36.
Lessard J, Sauvageau G . Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003; 423: 255–260.
Rizo A, Olthof S, Han L, Vellenga E, de Haan G, Schuringa JJ . Repression of BMI1 in normal and leukemic human CD34(+) cells impairs self-renewal and induces apoptosis. Blood 2009; 114: 1498–1505.
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest 2009; 119: 3626–3636.
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol 2010; 12: 982–992.
Lin X, Ojo D, Wei F, Wong N, Gu Y, Tang D . A novel aspect of tumorigenesis-BMI1 functions in regulating DNA damage response. Biomolecules 2015; 5: 3396–3415.
Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009; 459: 387–392.
Ginjala V, Nacerddine K, Kulkarni A, Oza J, Hill SJ, Yao M et al. BMI1 is recruited to DNA breaks and contributes to DNA damage-induced H2A ubiquitination and repair. Mol Cell Biol 2011; 31: 1972–1982.
Nacerddine K, Beaudry JB, Ginjala V, Westerman B, Mattiroli F, Song JY et al. Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J Clin Invest 2012; 122: 1920–1932.
Dimri M, Kang M, Dimri GP . A miR-200c/141-BMI1 autoregulatory loop regulates oncogenic activity of BMI1 in cancer cells. Oncotarget 2016; 7: 36220–36234.
Kufe D . Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 2009; 9: 874–885.
Kufe D . MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene 2013; 32: 1073–1081.
Raina D, Uchida Y, Kharbanda A, Rajabi H, Panchamoorthy G, Jin C et al. Targeting the MUC1-C oncoprotein downregulates HER2 activation and abrogates trastuzumab resistance in breast cancer cells. Oncogene 2014; 33: 3422–3431.
Kharbanda A, Rajabi H, Jin C, Tchaicha J, Kikuchi E, Wong K et al. Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clin Cancer Res 2014; 20: 5423–5434.
Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi M et al. MUC1-C oncoprotein induces TCF7L2 transcription factor activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem 2012; 287: 10703–10713.
Bouillez A, Rajabi H, Pitroda S, Jin C, Alam M, Kharbanda A et al. Inhibition of MUC1-C suppresses MYC expression and attenuates malignant growth in KRAS mutant lung adenocarcinomas. Cancer Res 2016; 76: 1538–1548.
Tagde A, Rajabi H, Bouillez A, Alam M, Gali R, Bailey S et al. MUC1-C drives MYC in multiple myeloma. Blood 2016; 127: 2587–2597.
Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S et al. MUC1 oncoprotein activates the IκB kinase β complex and constitutive NF-κB signaling. Nat Cell Biol 2007; 9: 1419–1427.
Ahmad R, Raina D, Joshi MD, Kawano T, Kharbanda S, Kufe D . MUC1-C oncoprotein functions as a direct activator of the NF-κB p65 transcription factor. Cancer Res 2009; 69: 7013–7021.
Takahashi H, Jin C, Rajabi H, Pitroda S, Alam M, Ahmad R et al. MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene 2015; 34: 5187–5197.
Rajabi H, Alam M, Takahashi H, Kharbanda A, Guha M, Ahmad R et al. MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene 2014; 33: 1680–1689.
Alam M, Ahmad R, Rajabi H, Kufe D . MUC1-C induces the LIN28B→LET-7→HMGA2 axis and self-renewal in NSCLC cells. Mol Cancer Res 2015; 13: 449–460.
Alam M, Rajabi H, Ahmad R, Jin C, Kufe D . Targeting the MUC1-C oncoprotein inhibits self-renewal capacity of breast cancer cells. Oncotarget 2014; 5: 2622–2634.
Kharbanda A, Rajabi H, Jin C, Alam M, Wong K, Kufe D . MUC1-C confers EMT and KRAS independence in mutant KRAS lung cancer cells. Oncotarget 2014; 5: 8893–8905.
Tam WL, Weinberg RA . The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013; 19: 1438–1449.
Rajabi H, Tagde A, Alam M, Bouillez A, Pitroda S, Suzuki Y et al. DNA methylation by DNMT1 and DNMT3b methyltransferases is driven by the MUC1-C oncoprotein in human carcinoma cells. Oncogene e-pub ahead of print 23 May 2016 doi:10.1038/onc.2016.180.
Tagde A, Rajabi H, Stroopinsky D, Gali R, Alam M, Bouillez A et al. MUC1-C induces DNA methyltransferase 1 and represses tumor suppressor genes in acute myeloid leukemia. Oncotarget 2016; 7: 38974–38987.
Kufe D . Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther 2009; 8: 1201–1207.
Raina D, Ahmad R, Rajabi H, Panchamoorthy G, Kharbanda S, Kufe D . Targeting cysteine-mediated dimerization of the MUC1-C oncoprotein in human cancer cells. Int J Oncol 2012; 40: 1643–1649.
Raina D, Agarwal P, Lee J, Bharti A, McKnight C, Sharma P et al. Characterization of the MUC1-C cytoplasmic domain as a cancer target. PLoS One 2015; 10: e0135156.
Huang R, Cheung NK, Vider J, Cheung IY, Gerald WL, Tickoo SK et al. MYCN and MYC regulate tumor proliferation and tumorigenesis directly through BMI1 in human neuroblastomas. FASEB J 2011; 25: 4138–4149.
Alam M, Bouillez A, Tagde A, Ahmad R, Rajabi H, Maeda T et al. MUC1‐C represses the crumbs complex polarity factor CRB3 and downregulates the Hippo pathway. Mol Cancer Res e-pub ahead of print 22 Spetember 2016; pii: molcanres.0233.2016.
Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009; 138: 592–603.
Wei J, Zhai L, Xu J, Wang H . Role of Bmi1 in H2A ubiquitylation and Hox gene silencing. J Biol Chem 2006; 281: 22537–22544.
Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M . Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev 1999; 13: 2678–2690.
Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M . The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 1999; 397: 164–168.
Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev 2007; 21: 525–530.
Meng S, Luo M, Sun H, Yu X, Shen M, Zhang Q et al. Identification and characterization of Bmi-1-responding element within the human p16 promoter. J Biol Chem 2010; 285: 33219–33229.
Campisi J . Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 2005; 120: 513–522.
Polyak K, Weinberg RA . Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9: 265–273.
Alam M, Ahmad R, Rajabi H, Kharbanda A, Kufe D . MUC1-C oncoprotein activates ERK→C/EBPβ-mediated induction of aldehyde dehydrogenase activity in breast cancer cells. J Biol Chem 2013; 288: 30829–30903.
Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M . Stem cells and cancer; the polycomb connection. Cell 2004; 118: 409–418.
Cho JH, Dimri M, Dimri GP . A positive feedback loop regulates the expression of polycomb group protein BMI1 via WNT signaling pathway. J Biol Chem 2013; 288: 3406–3418.
Yamamoto M, Bharti A, Li Y, Kufe D . Interaction of the DF3/MUC1 breast carcinoma-associated antigen and β-catenin in cell adhesion. J Biol Chem 1997; 272: 12492–12494.
Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D . MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res 2005; 65: 10413–10422.
Fleming A, Osley MA . Silence of the rings. Cell 2004; 119: 449–451.
Mallo M, Alonso CR . The regulation of Hox gene expression during animal development. Development 2013; 140: 3951–3963.
Endoh M, Endo TA, Endoh T, Isono K, Sharif J, Ohara O et al. Histone H2A mono-ubiquitination is a crucial step to mediate PRC1-dependent repression of developmental genes to maintain ES cell identity. PLoS Genet 2012; 8: e1002774.
Kazanets A, Shorstova T, Hilmi K, Marques M, Witcher M . Epigenetic silencing of tumor suppressor genes: paradigms, puzzles, and potential. Biochim Biophys Acta 2016; 1865: 275–288.
Hasegawa M, Sinha RK, Kumar M, Alam M, Yin L, Raina D et al. Intracellular targeting of the oncogenic MUC1-C protein with a novel GO-203 nanoparticle formulation. Clin Cancer Res 2015; 21: 2338–2347.
Hiraki M, Suzuki Y, Alam M, Hinohara K, Hasegawa M, Jin C et al. MUC1-C stabilizes MCL-1 in the oxidative stress response of triple-negative breast cancer cells to BCL-2 inhibitors. Sci Rep 2016; 6: 26643.
Hasegawa M, Takahashi H, Rajabi H, Alam M, Suzuki Y, Yin L et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget 2016; 7: 11756–11769.
Hiraki M, Nishimura J, Takahashi H, Wu X, Takahashi Y, Miyo M et al. Concurrent targeting of KRAS and AKT by MiR-4689 is a novel treatment against mutant KRAS colorectal cancer. Mol Ther Nucleic Acids 2015; 4: e231.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Acknowledgements
This work was supported by Grants from the National Cancer Institute of the National Institutes of Health under award numbers CA97098 and CA166480 and by the Lung Cancer Research Foundation. The authors thank Dr Goberdhan P Dimri, Department of Biochemistry and Molecular Medicine, School of Medicine and Health Sciences, The George Washington University, Washington, DC for kindly providing the pGL-BMI1PrWT and pGL-BMI1PrMut reporter vectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DK holds equity in Genus Oncology and is a consultant to the company. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hiraki, M., Maeda, T., Bouillez, A. et al. MUC1-C activates BMI1 in human cancer cells. Oncogene 36, 2791–2801 (2017). https://doi.org/10.1038/onc.2016.439
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.439
This article is cited by
-
XIST and MUC1-C form an auto-regulatory pathway in driving cancer progression
Cell Death & Disease (2024)
-
MUC1-C regulates NEAT1 lncRNA expression and paraspeckle formation in cancer progression
Oncogene (2024)
-
MUC1-C intersects chronic inflammation with epigenetic reprogramming by regulating the set1a compass complex in cancer progression
Communications Biology (2023)
-
Addiction of Merkel cell carcinoma to MUC1-C identifies a potential new target for treatment
Oncogene (2022)
-
The multifaceted role of MUC1 in tumor therapy resistance
Clinical and Experimental Medicine (2022)