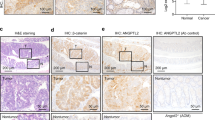
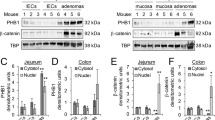
Abstract
Nedd4 (Nedd4-1) is an E3 ubiquitin ligase that belongs to the HECT family and comprises a C2-WW(n)-HECT domain architecture. Although it has been reported to regulate growth factor receptors and cellular signaling, its role in cancer development has been controversial, with some studies proposing that it promotes cancer while others suggest it inhibits tumor growth. Here, we tested the effect of Nedd4 on intestinal tumor formation and growth using Nedd4-knockout mice (Nedd4 floxed (fl) mice crossed to villin-Cre mice). Although we find that knockout of Nedd4 on its own does not cause tumor growth, its knockout in the context of Apc+/min-derived colorectal tumors leads to augmentation of tumor growth, suggesting that Nedd4 normally suppresses intestinal WNT signaling and growth of colonic tumors. WNT signaling microarray, immunoblotting and immunohistochemistry analyses of tumors derived from the Villin-Cre;Nedd4fl/fl;Apc+/min colons demonstrated elevated expression of the WNT upstream effectors LEF1 (full length) and YY1 in these tumors relative to control (Apc+/min alone) tumors. Together, these results suggest that Nedd4 suppresses colonic WNT signaling and tumor growth, at least in part, by suppressing the transcription factors LEF1 and YY1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Klaus A, Birchmeier W . Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008; 8: 387–398.
Lucero OM, Dawson DW, Moon RT, Chien AJ . A re-evaluation of the "oncogenic" nature of Wnt/beta-catenin signaling in melanoma and other cancers. Curr Oncol Rep 2010; 12: 314–318.
Najdi R, Holcombe RF, Waterman ML . Wnt signaling and colon carcinogenesis: beyond APC. J Carcinog 2011; 10: 5.
Barker N, Bartfeld S, Clevers H . Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell 2010; 7: 656–670.
van Noort M, Clevers H . TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev Biol 2002; 244: 1–8.
Aberle H, Bauer A, Stappert J, Kispert A, Kemler R . Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 1997; 16: 3797–3804.
Cadigan KM, Waterman ML . TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol 2012; 4: 6–14.
Wend P, Holland JD, Ziebold U, Birchmeier W . Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol 2010; 21: 855–863.
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457: 608–611.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 2011; 469: 415–418.
Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12: 468–476.
Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature 2014; 506: 52–57.
Rotin D, Kumar S . Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 2009; 10: 398–409.
Huang Z, Choi BK, Mujoo K, Fan X, Fa M, Mukherjee S et al. The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling. Oncogene 2015; 34: 1105–1115.
Katz M, Shtiegman K, Tal-Or P, Yakir L, Mosesson Y, Harari D et al. Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic 2002; 3: 740–751.
Murdaca J, Treins C, Monthouel-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E et al. Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem 2004; 279: 26754–26761.
Persaud A, Alberts P, Hayes M, Guettler S, Clarke I, Sicheri F et al. Nedd4-1 binds and ubiquitylates activated FGFR1 to control its endocytosis and function. EMBO J 2011; 30: 3259–3273.
Yang B, Gay DL, MacLeod MK, Cao X, Hala T, Sweezer EM et al. Nedd4 augments the adaptive immune response by promoting ubiquitin-mediated degradation of Cbl-b in activated T cells. Nat Immunol 2008; 9: 1356–1363.
Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP . A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci USA 2000; 97: 13871–13876.
Leykauf K, Salek M, Bomke J, Frech M, Lehmann WD, Durst M et al. Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J Cell Sci 2006; 119: 3634–3642.
Fouladkou F, Lu C, Jiang C, Zhou L, She Y, Walls JR et al. The ubiquitin ligase Nedd4-1 is required for heart development and is a suppressor of thrombospondin-1. J Biol Chem 2010; 285: 6770–6780.
Ing B, Shteiman-Kotler A, Castelli M, Henry P, Pak Y, Stewart B et al. Regulation of Commissureless by the ubiquitin ligase DNedd4 is required for neuromuscular synaptogenesis in Drosophila melanogaster. Mol Cell Biol 2007; 27: 481–496.
Liu Y, Oppenheim RW, Sugiura Y, Lin W . Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Dev Biol 2009; 330: 153–166.
Hsia HE, Kumar R, Luca R, Takeda M, Courchet J, Nakashima J et al. Ubiquitin E3 ligase Nedd4-1 acts as a downstream target of PI3K/PTEN-mTORC1 signaling to promote neurite growth. Proc Natl Acad Sci USA 2014; 111: 13205–13210.
Plant PJ, Yeger H, Staub O, Howard P, Rotin D . The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J Biol Chem 1997; 272: 32329–32336.
Persaud A, Alberts P, Mari S, Tong J, Murchie R, Maspero E et al. Tyrosine phosphorylation of NEDD4 activates its ubiquitin ligase activity. Sci Signal 2014; 7: ra95.
Wang J, Peng Q, Lin Q, Childress C, Carey D, Yang W . Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J Biol Chem 2010; 285: 12279–12288.
Wiesner S, Ogunjimi AA, Wang HR, Rotin D, Sicheri F, Wrana JL et al. Autoinhibition of the HECT-type ubiquitin ligase Smurf2 through its C2 domain. Cell 2007; 130: 651–662.
Kanelis V, Rotin D, Forman-Kay JD . Solution structure of a Nedd4 WW domain-ENaC peptide complex. Nat Struct Biol 2001; 8: 407–412.
Kanelis V, Bruce MC, Skrynnikov NR, Rotin D, Forman-Kay JD . Structural determinants for high-affinity binding in a Nedd4 WW3* domain-Comm PY motif complex. Structure 2006; 14: 543–553.
March HN, Rust AG, Wright NA, ten Hoeve J, de Ridder J, Eldridge M et al. Insertional mutagenesis identifies multiple networks of cooperating genes driving intestinal tumorigenesis. Nat Genet 2011; 43: 1202–1209.
Martin ES, Tonon G, Sinha R, Xiao Y, Feng B, Kimmelman AC et al. Common and distinct genomic events in sporadic colorectal cancer and diverse cancer types. Cancer Res 2007; 67: 10736–10743.
Eide PW, Cekaite L, Danielsen SA, Eilertsen IA, Kjenseth A, Fykerud TA et al. NEDD4 is overexpressed in colorectal cancer and promotes colonic cell growth independently of the PI3K/PTEN/AKT pathway. Cell Signal 2013; 25: 12–18.
Kim SS, Yoo NJ, Jeong EG, Kim MS, Lee SH . Expression of NEDD4-1, a PTEN regulator, in gastric and colorectal carcinomas. APMIS 2008; 116: 779–784.
Tanksley JP, Chen X, Coffey RJ . NEDD4L is downregulated in colorectal cancer and inhibits canonical WNT signaling. PLoS One 2013; 8: e81514.
Kawabe H, Neeb A, Dimova K, Young SM Jr., Takeda M, Katsurabayashi S et al. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron 2010; 65: 358–372.
el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 2004; 39: 186–193.
Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL . Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 2002; 277: 33275–33283.
Fodde R, Smits R, Clevers H . APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer 2001; 1: 55–67.
Moser AR, Pitot HC, Dove WF . A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science (New York, NY) 1990; 247: 322–324.
Arce L, Yokoyama NN, Waterman ML . Diversity of LEF/TCF action in development and disease. Oncogene 2006; 25: 7492–7504.
Hovanes K, Li TW, Munguia JE, Truong T, Milovanovic T, Lawrence Marsh J et al. Beta-catenin-sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat Genet 2001; 28: 53–57.
Porfiri E, Rubinfeld B, Albert I, Hovanes K, Waterman M, Polakis P . Induction of a beta-catenin-LEF-1 complex by wnt-1 and transforming mutants of beta-catenin. Oncogene 1997; 15: 2833–2839.
Wang WJ, Yao Y, Jiang LL, Hu TH, Ma JQ, Liao ZJ et al. Knockdown of lymphoid enhancer factor 1 inhibits colon cancer progression in vitro and in vivo. PLoS One 2013; 8: e76596.
Chinnappan D, Xiao D, Ratnasari A, Andry C, King TC, Weber HC . Transcription factor YY1 expression in human gastrointestinal cancer cells. Int J Oncol 2009; 34: 1417–1423.
Yokoyama NN, Pate KT, Sprowl S, Waterman ML . A role for YY1 in repression of dominant negative LEF-1 expression in colon cancer. Nucleic Acids Res 2010; 38: 6375–6388.
Zaravinos A, Spandidos DA . Yin yang 1 expression in human tumors. Cell Cycle 2010; 9: 512–522.
Persaud A, Alberts P, Amsen EM, Xiong X, Wasmuth J, Saadon Z et al. Comparison of substrate specificity of the ubiquitin ligases Nedd4 and Nedd4-2 using proteome arrays. Mol Syst Biol 2009; 5: 333.
Owens BM, Simmons A . Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol 2013; 6: 224–234.
Pinchuk IV, Mifflin RC, Saada JI, Powell DW . Intestinal mesenchymal cells. Curr Gastroenterol Rep 2010; 12: 310–318.
Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW . Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res 2004; 10: 5870–5879.
Buller NV, Rosekrans SL, Metcalfe C, Heijmans J, van Dop WA, Fessler E et al. Stromal Indian hedgehog signaling is required for intestinal adenoma formation in mice. Gastroenterology 2015; 148: 170–180 e176.
Isella C, Terrasi A, Bellomo SE, Petti C, Galatola G, Muratore et al. Stromal contribution to the colorectal cancer transcriptome. Nat Genet 2015; 47: 312–319.
Pali-Scholl I, Yildirim AO, Ackermann U, Knauer T, Becker C, Garn H et al. Anti-acids lead to immunological and morphological changes in the intestine of BALB/c mice similar to human food allergy. Exp Toxicol Pathol 2008; 60: 337–345.
Moolenbeek C, Ruitenberg EJ . The "Swiss roll": a simple technique for histological studies of the rodent intestine. Lab Animals 1981; 15: 57–59.
Acknowledgements
We would like to thank Kazuko Hay for technical support and Dr Sylvie Robine for the Vil-CreERT2 mice. This grant was supported by the Canadian Institute of Health Research (MOP-130422, to DR), the German Research Foundation SPP1365/KA3423/1-1 (to HK) and the Fritz Thyssen Foundation (to HK). DR is a recipient of a Canada Research Chair form the Canadian Foundation for Innovation (CFI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, C., Thoeni, C., Connor, A. et al. Intestinal knockout of Nedd4 enhances growth of Apcmin tumors. Oncogene 35, 5839–5849 (2016). https://doi.org/10.1038/onc.2016.125
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.125
This article is cited by
-
NEDD4 regulates ubiquitination and stability of the cell adhesion molecule IGPR-1 via lysosomal pathway
Journal of Biomedical Science (2021)
-
NEDD4 expression is associated with breast cancer progression and is predictive of a poor prognosis
Breast Cancer Research (2019)
-
The many substrates and functions of NEDD4-1
Cell Death & Disease (2019)