Abstract
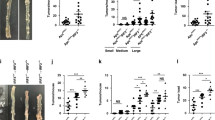
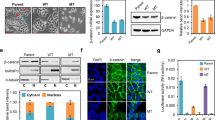
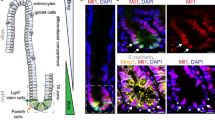
Uncontrolled proliferation of intestinal epithelial cells caused by mutations in genes of the WNT/β-catenin pathway is associated with development of intestinal cancers. We previously reported that intestinal stromal cell-derived angiopoietin-like protein 2 (ANGPTL2) controls epithelial regeneration and intestinal immune responses. However, the role of tumor cell-derived ANGPTL2 in intestinal tumorigenesis remained unclear. Here, we show that tumor cell-derived ANGPTL2 promotes β-catenin-driven intestinal tumorigenesis. ANGPTL2 deficiency suppressed intestinal tumor development in an experimental mouse model of sporadic colon cancer. We also found that increased ANGPTL2 expression in colorectal cancer (CRC) cells augments β-catenin pathway signaling and promotes tumor cell proliferation. Relevant to mechanism, our findings suggest that tumor cell-derived ANGPTL2 upregulates expression of OB-cadherin, which then interacts with β-catenin, blocking destruction complex-independent proteasomal degradation of β-catenin proteins. Moreover, our observations support a model whereby ANGPTL2-induced OB-cadherin expression in CRC cells is accompanied by decreased cell surface integrin α5β1 expression. These findings overall provide novel insight into mechanisms of β-catenin-driven intestinal tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kimelman D, Xu W. β-Catenin destruction complex: Insights and questions from a structural perspective. Oncogene. 2006;25:7482–91.
Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: Diseases and therapies. Nat Rev Genet. 2004;5:691–701.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. 434, 843–850 (2005). Nature. 2005;434:843–50.
Kumawat K, Koopmans T, Gosens R. Β-Catenin As a Regulator and Therapeutic Target for Asthmatic Airway Remodeling. Expert Opin Ther Targets. 2014;18:1023–34.
Hato T, Tabata M, Oike Y. The Role of Angiopoietin-Like Proteins in Angiogenesis and Metabolism. Trends Cardiovasc. Med. 2008;18. https://doi.org/10.1016/j.tcm.2007.10.003.
Oike Y, Yasunaga K, Ito Y, Matsumoto S ichiro, Maekawa H, Morisada T, et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration. Proc Natl Acad Sci USA 2003;100. https://doi.org/10.1073/pnas.1531901100.
Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab. 2014;25:245–54.
Horiguchi H, Endo M, Kawane K, Kadomatsu T, Terada K, Morinaga J, et al. ANGPTL2 expression in the intestinal stem cell niche controls epithelial regeneration and homeostasis. EMBO J. 2017;36:409–24.
Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, et al. Angiopoietin-like Protein 2 Promotes Chronic Adipose Tissue Inflammation and Obesity-Related Systemic Insulin Resistance. Cell Metab. 2009;10:178–88.
Endo M, Nakano M, Kadomatsu T, Fukuhara S, Kuroda H, Mikami S, et al. Tumor Cell-Derived Angiopoietin-like Protein ANGPTL2 Is a Critical Driver of Metastasis. Cancer Res. 2012;72:1784–94.
Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, et al. The Secreted Protein ANGPTL2 Promotes Metastasis of Osteosarcoma Cells Through Integrin 5 1, p38 MAPK, and Matrix Metalloproteinases. Sci Signal. 2014;7:ra7.
Takahashi M, Fukuda K, Sugimura T, Wakabayashi K. Β-Catenin Is Frequently Mutated and Demonstrates Altered Cellular Location in Azoxymethane-Induced Rat Colon Tumors. Cancer Res. 1998;58:42–46.
Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54.
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991;253. https://doi.org/10.1126/science.1651563.
Su YY, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, et al. APC Is Essential for Targeting Phosphorylated β-Catenin to the SCFβ-TrCP Ubiquitin Ligase. Mol Cell. 2008;32. https://doi.org/10.1016/j.molcel.2008.10.023.
Li VSW, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt Signaling through Inhibition of β-Catenin Degradation in an Intact Axin1 Complex. Cell. 2012;149. https://doi.org/10.1016/j.cell.2012.05.002.
Osumi H, Horiguchi H, Kadomatsu T, Tashiro K, Morinaga J, Takahashi T, et al. Tumor cell-derived angiopoietin-like protein 2 establishes a preference for glycolytic metabolism in lung cancer cells. Cancer Sci. 2020;111:1241–53.
Hoffmann I, Balling R. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev Biol. 1995;169:337–46.
Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, et al. Integrinα5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8. https://doi.org/10.1016/j.devcel.2005.03.006.
Starchenko A, Graves-Deal R, Yang YP, Li C, Zent R, Singh B, et al. Clustering of integrin α5 at the lateral membrane restores epithelial polarity in invasive colorectal cancer cells. Mol Biol Cell. 2017;28. https://doi.org/10.1091/mbc.E16-12-0852.
Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70.
Kuhnert F, Davis CR, Wang HT, Chu P, Lee M, Yuan J, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci USA. 2004;101:266–71.
Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA. 1992;89:4452–6.
Kinzler KW, Vogelstein B Lessons from hereditary colorectal cancer. Cell. 1996;87. https://doi.org/10.1016/S0092-8674(00)81333-1.
Barker N, Ridgway RA, Van Es JH, Van De Wetering M, Begthel H, Van Den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11.
Okazaki M, Takeshita S, Kawai S, Kikuno R, Tsujimura A, Kudo A, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994;269:12092–8.
Shibata T, Ochiai A, Gotoh M, Machinami R, Hirohashi S. Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett. 1996;99:147–53.
Feltes CM, Kudo A, Blaschuk O, Byers SW. An alternatively spliced cadherin-11 enhances human breast cancer cell invasion. Cancer Res. 2002;62:6688–97.
Tomita K, Van Bokhoven A, Van Leenders GJLH, Ruijter ETG, Jansen CFJ, Bussemakers MJG, et al. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60:3650–4.
Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–52.
Munro SB, Turner JM, Farookhi R, Blaschuk OW, Jothy S. E-cadherin and ob-cadherin mrna levels in normal human colon and colon carcinoma. Exp Mol Pathol. 1995;62:118–22.
Kaur H, Phillips-Mason PJ, Burden-Gulley SM, Kerstetter-Fogle AE, Basilion JP, Sloan AE, et al. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res. 2012;10. https://doi.org/10.1158/1541-7786.MCR-11-0457.
Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ, Chen DT, et al. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res. 2008;6. https://doi.org/10.1158/1541-7786.MCR-08-0077.
Zhu Q, Wang Z, Zhou L, Ren Y, Gong Y, Qin W, et al. The role of cadherin-11 in microcystin-LR-induced migration and invasion in colorectal carcinoma cells. Oncol Lett. 2018;15:1417–22.
Satriyo P, Bamodu O, Chen J-H, Aryandono T, Haryana S, Yeh C-T, et al. Cadherin 11 Inhibition Downregulates β-catenin, Deactivates the Canonical WNT Signalling Pathway and Suppresses the Cancer Stem Cell-Like Phenotype of Triple Negative Breast Cancer. J Clin Med. 2019;8:148.
Chen JH, Huang WC, Bamodu OA, Chang PMH, Chao TY, Huang TH. Monospecific antibody targeting of CDH11 inhibits epithelial-to-mesenchymal transition and represses cancer stem cell-like phenotype by up-regulating miR-335 in metastatic breast cancer, in vitro and in vivo. BMC Cancer. 2019;19:1–13.
Zeisberg M, Neilson EG Biomarkers for epithelial-mesenchymal transitions. J Clin Investig. 2009;119. https://doi.org/10.1172/JCI36183.
Halbleib JM, Sääf AM, Brown PO, Nelson WJ. Transcriptional modulation of genes encoding structural characteristics of differentiating enterocytes during development of a polarized epithelium in vitro. Mol Biol Cell. 2007;18. https://doi.org/10.1091/mbc.E07-04-0308.
Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, et al. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71:7502–12.
Waite KA, Eng C From developmental disorder to heritable cancer: It’s all in the BMP/TGF-β family. Nat Rev Genet. 2003;4. https://doi.org/10.1038/nrg1178.
Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, et al. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28. https://doi.org/10.1038/88919.
Van Es JH, Giles RH, Clevers HC. The many faces of the tumor suppressor gene APC. Exp Cell Res. 2001; 264. https://doi.org/10.1006/excr.2000.5142.
Masuda T, Endo M, Yamamoto Y, Odagiri H, Kadomatsu T, Nakamura T, et al. ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling. Sci Rep. 2015;5:9170.
Horiguchi H, Endo M, Miyamoto Y, Sakamoto Y, Odagiri H, Masuda T, et al. Angiopoietin-like protein 2 renders colorectal cancer cells resistant to chemotherapy by activating spleen tyrosine kinase-phosphoinositide 3-kinase-dependent anti-apoptotic signaling. Cancer Sci. 2014;105:1550–9.
Horiguchi H, Kadomatsu T, Kurahashi R, Hara C, Miyata K, Baba M, et al. Dual functions of angiopoietin-like protein 2 signaling in tumor progression and anti-tumor immunity. Genes Dev. 2019;33:1641–56.
Horiguchi H, Kadomatsu T, Miyata K, Terada K, Sato M, Torigoe D, et al. Stroma-derived ANGPTL2 establishes an anti-tumor microenvironment during intestinal tumorigenesis. Oncogene. 2021;40:55–67.
Motokawa I, Endo M, Terada K, Horiguchi H, Miyata K, Kadomatsu T, et al. Interstitial pneumonia induced by bleomycin treatment is exacerbated in Angptl2-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2016;311:L704–L713.
Oudhoff MJ, Braam MJS, Freeman SA, Wong D, Rattray DG, Wang J, et al. SETD7 Controls Intestinal Regeneration and Tumorigenesis by Regulating Wnt/β-Catenin and Hippo/YAP Signaling. Dev Cell. 2016;37:47–57.
Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2. https://doi.org/10.1038/nprot.2007.261.
Rappsilber J, Ishihama Y, Mann M Stop And Go Extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75. https://doi.org/10.1021/ac026117i.
Acknowledgements
We thank Kiyoka Tabu, Noriko Shirai, and Sayomi Iwaki for technical assistance. This work was supported by the Scientific Research Fund of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (grant 21K07101 to TK and grant 21K15508 to HH), the Takeda Science Foundation (HH, TK), and Tasaki Memorial Research Grant for 2021 (HH).
Author information
Authors and Affiliations
Contributions
HH designed the study, performed and analyzed most of experiments, and wrote the paper. TK designed the study and wrote the paper. SYumoto and HB collected human data. TMasuda and SO performed proteomics. KM provided Angptl2 mutant mice. SYamamura, MS, and JM discussed the data. TMoroishi designed and supervised the study. YO coordinated, designed, and supervised the study, and wrote the paper. All authors discussed the data and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horiguchi, H., Kadomatsu, T., Yumoto, S. et al. Tumor cell-derived ANGPTL2 promotes β-catenin-driven intestinal tumorigenesis. Oncogene 41, 4028–4041 (2022). https://doi.org/10.1038/s41388-022-02405-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02405-8
This article is cited by
-
Tumor stroma-derived ANGPTL2 potentiates immune checkpoint inhibitor efficacy
Cancer Gene Therapy (2024)
-
ANGPTL2 promotes immune checkpoint inhibitor-related murine autoimmune myocarditis
Communications Biology (2023)