Abstract
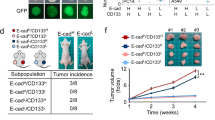
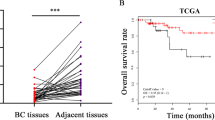
Claudin-low tumors are a highly aggressive breast cancer subtype with no targeted treatments and a clinically documented resistance to chemotherapy. They are significantly enriched in cancer stem cells (CSCs), which makes claudin-low tumor models particularly attractive for studying CSC behavior and developing novel approaches to minimize CSC therapy resistance. One proposed mechanism by which CSCs arise is via an epithelial–mesenchymal transition (EMT), and reversal of this process may provide a potential therapeutic approach for increasing tumor chemosensitivity. Therefore, we investigated the role of known EMT regulators, miR-200 family of microRNAs in controlling the epithelial state, stem-like properties and therapeutic response in an in vivo primary, syngeneic p53null claudin-low tumor model that is normally deficient in miR-200 expression. Using an inducible lentiviral approach, we expressed the miR-200c cluster in this model and found that it changed the epithelial state, and consequently, impeded CSC behavior in these mesenchymal tumors. Moreover, these state changes were accompanied by a decrease in proliferation and an increase in the differentiation status. miR-200c expression also forced a significant reorganization of tumor architecture, affecting important cellular processes involved in cell–cell contact, cell adhesion and motility. Accordingly, induced miR200c expression significantly enhanced the chemosensitivity and decreased the metastatic potential of this p53null claudin-low tumor model. Collectively, our data suggest that miR-200c expression in claudin-low tumors offers a potential therapeutic application to disrupt the EMT program on multiple fronts in this mesenchymal tumor subtype, by altering tumor growth, chemosensitivity and metastatic potential in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 2010; 12: R68.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Thiery JP, Acloque H, Huang RYJ, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Hennessy BT, Gonzalez-Angulo A-M, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee J-S et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res 2009; 69: 4116–4124.
Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA 2010; 107: 15449–15454.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA 2009; 106: 13820–13825.
Creighton CJ, Chang JC, Rosen JM . Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia 2010; 15: 253–260.
Visvader JE, Lindeman GJ . Cancer stem cells: Current status and evolving complexities. Cell Stem Cell 2012; 10: 717–728.
Kim N-G, Koh E, Chen X, Gumbiner BM . E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA 2011; 108: 11930–11935.
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA . Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 2008; 68: 3645–3654.
Brabletz S, Brabletz T . The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 2010; 11: 670–677.
Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell 2011; 22: 1686–1698.
Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009; 138: 592–603.
Lim Y-Y, Wright JA, Attema JL, Gregory PA, Bert AG, Smith E et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci 2013; 126: 2256–2266.
Hurteau GJ, Carlson JA, Roos E, Brock GJ . Stable expression of miR-200c alone is sufficient to regulate TCF8 (ZEB1) and restore E-cadherin expression. Cell Cycle 2009; 8: 2064–2069.
Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celià-Terrassa T et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med 2011; 17: 1101–1108.
Jerry D, Kittrell F, Kuperwasser C, Laucirica R, Dickinson E, Bonilla P et al. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene 2000; 19: 1052–1058.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Herschkowitz JI, Zhao W, Zhang M, Usary J, Murrow G, Edwards D et al. Comparative oncogenomics identifies breast tumors enriched in functional tumor-initiating cells. Proc Natl Acad Sci USA 2012; 109: 2778–2783.
Pfefferle AD, Herschkowitz JI, Usary J, Harrell JC, Spike BT, Adams JR et al. Transcriptomic classification of genetically engineered mouse models of breast cancer identifies human subtype counterparts. Genome Biol 2013; 14: R125.
Zhang M, Behbod F, Atkinson RL, Landis MD, Kittrell F, Edwards D et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res 2008; 68: 4674–4682.
Howe EN, Cochrane DR, Richer JK . Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res 2011; 13: R45.
Jurmeister S, Baumann M, Balwierz A, Keklikoglou I, Ward A, Uhlmann S et al. MicroRNA-200c represses migration and invasion of breast cancer cells by targeting actin-regulatory proteins FHOD1 and PPM1F. Mol Cell Biol 2012; 32: 633–651.
Meerbrey KL, Hu G, Kessler JD, Roarty K, Li MZ, Fang JE et al. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci USA 2011; 108: 3665–3670.
Usary J, Zhao W, Darr D, Roberts PJ, Liu M, Balletta L et al. Predicting drug responsiveness in human cancers using genetically engineered mice. Clin Cancer Res 2013; 19: 4889–4899.
Castedo M, Perfettini J-L, Roumier T, Andreau K, Medema R, Kroemer G . Cell death by mitotic catastrophe: a molecular definition. Oncogene 2004; 23: 2825–2837.
Folkman J . Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29: 15–18.
Folkman J . Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–1186.
Weidner N, Semple JP, Welch WR, Folkman J . Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8.
Pecot C V, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu C et al. Tumour angiogenesis regulation by the miR-200 family. Nat Commun 2013; 4: 2427.
Roybal JD, Zang Y, Ahn Y-H, Yang Y, Gibbons DL, Baird BN et al. miR-200 Inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol Cancer Res 2011; 9: 25–35.
Jechlinger M, Sommer A, Moriggl R, Seither P, Kraut N, Capodiecci P et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest 2006; 116: 1561–1570.
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008; 10: 593–601.
Park S-M, Gaur AB, Lengyel E, Peter ME . The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008; 22: 894–907.
Visvader JE . Cells of origin in cancer. Nature 2011; 469: 314–322.
Chuthapisith S, Eremin J, El-Sheemey M, Eremin O . Breast cancer chemoresistance: Emerging importance of cancer stem cells. Surg Oncol 2010; 19: 27–32.
Pinto CA, Widodo E, Waltham M, Thompson EW . Breast cancer stem cells and epithelial mesenchymal plasticity - Implications for chemoresistance. Cancer Lett 2013; 341: 56–62.
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep 2014; 2: 78–91.
Cui J, Cheng Y, Zhang P, Sun M, Gao F, Liu C et al. Down regulation of miR200c promotes radiation-induced thymic lymphoma by targeting BMI1. J Cell Biochem 2014; 115: 1033–1042.
Kopp F, Oak PS, Wagner E, Roidl A . miR-200c sensitizes breast cancer cells to doxorubicin treatment by decreasing TrkB and Bmi1 expression. PLoS One 2012. 7.
Chang CJ, Yang JY, Xia W, Chen C, Te, Xie X, Chao CH et al. EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-β-catenin signaling. Cancer Cell 2011; 19: 86–100.
Yin J, Zheng G, Jia X, Zhang Z, Zhang W, Song Y et al. A Bmi1-miRNAs cross-talk modulates chemotherapy response to 5-fluorouracil in breast cancer cells. PLoS One 2013. 8.
Zhang P, Wei Y, Wang L, Debeb BG, Yuan Y, Zhang J et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat Cell Biol 2014; 16: 864–875.
Dykxhoorn DM, Wu Y, Xie H, Yu F, Lal A, Petrocca F et al. miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One 2009; 4: e7181.
Gravgaard KH, Lyng MB, Laenkholm AV, Søkilde R, Nielsen BS, Litman T et al. The miRNA-200 family and miRNA-9 exhibit differential expression in primary versus corresponding metastatic tissue in breast cancer. Breast Cancer Res Treat 2012; 134: 207–217.
Humphries B, Wang Z, Oom AL, Fisher T, Tan D, Cui Y et al. MicroRNA-200b targets protein kinase Cα and suppresses triple-negative breast cancer metastasis. Carcinogenesis 2014; 00: 1–10.
Wu SY, Lopez-Berestein G, Calin GA, Sood AK . RNAi therapies: drugging the undruggable. Sci Transl Med 2014; 6: 240ps7.
Welm BE, Dijkgraaf GJP, Bledau AS, Welm AL, Werb Z . Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell 2008; 2: 90–102.
Hu Y, Smyth GK . ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 2009; 347: 70–78.
Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol 2007; 8: R76.
Acknowledgements
These studies were supported by grants CA148761 (JMR and CP) from the National Cancer Institute and RP130485 (JMR) and RP140102 (JK) from CPRIT. This project was supported by the following core facilities and their funding: Biostatistics and Informatics (P30-CA125123) and Cytometry and Cell Sorting Core at Baylor College of Medicine (P30 AI036211, P30 CA125123 and S10 RR024574). RPPA arrays were performed at the MD Anderson Cancer Center RPPA Core Facility–Functional Proteomics. We thank Drs Kevin Roarty Amy Shore and Michael Toneff for helpful discussions and paper editing, Dr Jason Herschkowitz for help and advice, and Ms Shirley Small for animal husbandry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Knezevic, J., Pfefferle, A., Petrovic, I. et al. Expression of miR-200c in claudin-low breast cancer alters stem cell functionality, enhances chemosensitivity and reduces metastatic potential. Oncogene 34, 5997–6006 (2015). https://doi.org/10.1038/onc.2015.48
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.48
This article is cited by
-
Landscape of NcRNAs involved in drug resistance of breast cancer
Clinical and Translational Oncology (2023)
-
Ovarian toxicity of carboplatin and paclitaxel in mouse carriers of mutation in BRIP1 tumor suppressor gene
Scientific Reports (2022)
-
The miR-200 family in normal mammary gland development
BMC Developmental Biology (2021)
-
Re-expression of miR-200s in claudin‐low mammary tumor cells alters cell shape and reduces proliferation and invasion potentially through modulating other miRNAs and SUZ12 regulated genes
Cancer Cell International (2021)
-
ALG3 contributes to stemness and radioresistance through regulating glycosylation of TGF-β receptor II in breast cancer
Journal of Experimental & Clinical Cancer Research (2021)