Abstract
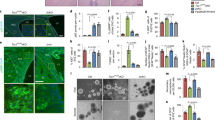
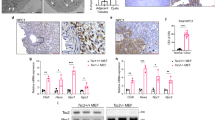
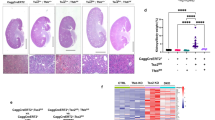
Functions of extracellular vesicles including exosomes in the pathogenesis of tuberous sclerosis complex (TSC) have not yet been studied. We report that the extracellular vesicles such as exosomes derived from tuberous sclerosis 1 (Tsc1)-null cells transform phenotypes of neighboring wild-type cells in vivo in such manner that they become functionally similar to Tsc1-null cells. The loss of Tsc1 in the mouse neural tube increases the number of the wild-type neuronal progenitors, which is followed by the precocious and transient acceleration of neuronal differentiation of these cells. The mechanisms regulating these changes involve the exosomal delivery of exosomal shuttle Notch1 and Rheb esRNA and component of γ-secretase complex presenilin 1 from Tsc1-null cells to wild-type cells leading to the activation of Notch and Rheb signaling in the recipient cells. The exosome-mediated mechanisms may also operate in the cells of angiomyolipoma (AML), which develops as a result of mutations in TSC1/TSC2 genes in TSC patients, because we observed the reactivation of mammalian target of rapamycin and Notch pathways, driven by the delivery of Rheb and Notch1 esRNA, in AML cells depleted of Rheb that were treated with the exosomes purified from AML cells with the constitutively high Rheb levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N et al. Terminology and classification of the cortical dysplasias. Neurology 2004; 62: S2–S8.
Wong M . Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia 2008; 49: 8–21.
Au KS, Hebert AA, Roach ES, Northrup H . Complete inactivation of the TSC2 gene leads to formation of hamartomas. Am J Hum Genet 1999; 65: 1790–1795.
Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ . Molecular genetic advances in tuberous sclerosis. Hum Genet 2000; 107: 97–114.
Ramesh V . Aspects of tuberous sclerosis complex (TSC) protein function in the brain. Biochem Soc Trans 2003; 31: 579–583.
Niida Y, Stemmer-Rachamimov AO, Logrip M, Tapon D, Perez R, Kwiatkowski DJ et al. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet 2001; 69: 493–503.
Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G 2nd et al. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol 2004; 56: 478–487.
Johnson MW, Emelin JK, Park SH, Vinters HV . Co-localization of TSC1 and TSC2 gene products in tubers of patients with tuberous sclerosis. Brain Pathol 1999; 9: 45–54.
Mizuguchi M, Ikeda K, Takashima S . Simultaneous loss of hamartin and tuberin from the cerebrum, kidney and heart with tuberous sclerosis. Acta Neuropathologica 2000; 99: 503–510.
Cepeda C, Andre VM, Yamazaki I, Hauptman JS, Chen JY, Vinters HV et al. Comparative study of cellular and synaptic abnormalities in brain tissue samples from pediatric tuberous sclerosis complex and cortical dysplasia type II. Epilepsia 2010; 51: 160–165.
Malatesta P, Hartfuss E, Gotz M . Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 2000; 127: 5253–5263.
Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR . Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001; 409: 714–720.
Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI et al. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol 2005; 194: 320–332.
Kornblum HI . Introduction to neural stem cells. Stroke 2007; 38: 810–816.
Lee C, Hu J, Ralls S, Kitamura T, Loh YP, Yang Y et al. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One 2012; 7: e50501.
Walker AS, Goings GE, Kim Y, Miller RJ, Chenn A, Szele FG . Nestin reporter transgene labels multiple central nervous system precursor cells. Neural Plasticity 2010; 2010: 894374.
Hockfield S, McKay RD . Identification of major cell classes in the developing mammalian nervous system. J Neurosci 1985; 5: 3310–3328.
Lendahl U, Zimmerman LB, McKay RD . CNS stem cells express a new class of intermediate filament protein. Cell 1990; 60: 585–595.
Martynoga B, Drechsel D, Guillemot F . Molecular control of neurogenesis: a view from the mammalian cerebral cortex. Cold Spring Harb Perspect Biol 2012; 4: 1–14.
Kim J, Lo L, Dormand E, Anderson DJ . SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 2003; 38: 17–31.
Okamura Y, Saga Y . Notch signaling is required for the maintenance of enteric neural crest progenitors. Development 2008; 135: 3555–3565.
Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D et al. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 2005; 118: 2849–2858.
Bakhti M, Winter C, Simons M . Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem 2011; 286: 787–796.
Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006; 20: 847–856.
Ge W, Martinowich K, Wu X, He F, Miyamoto A, Fan G et al. Notch signaling promotes astrogliogenesis via direct CSL-mediated glial gene activation. J Neurosci Res 2002; 69: 848–860.
Yu J, Astrinidis A, Howard S, Henske EP . Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol 2004; 286: L694–L700.
Baonza A, Freeman M . Notch signalling and the initiation of neural development in the Drosophila eye. Development 2001; 128: 3889–3898.
Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell 2014; 26: 707–721.
Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 2008; 358: 140–151.
Bonnet CS, Aldred M, von Ruhland C, Harris R, Sandford R, Cheadle JP . Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum Mol Genet 2009; 18: 2166–2176.
Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG Jr . TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 2003; 4: 147–158.
Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L et al. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci USA 2008; 105: 19384–19389.
Hall DJ, Grewal SS, de la Cruz AF, Edgar BA . Rheb-TOR signaling promotes protein synthesis, but not glucose or amino acid import, in Drosophila. BMC Biol 2007; 5: 10.
Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 2009; 18: 151–163.
Karbowniczek M, Robertson GP, Henske EP . Rheb inhibits C-raf activity and B-raf/C-raf heterodimerization. J Biol Chem 2006; 281: 25447–25456.
Karbowniczek M, Zitserman D, Khabibullin D, Hartman T, Yu J, Morrison T et al. The evolutionarily conserved TSC/Rheb pathway activates Notch in tuberous sclerosis complex and Drosophila external sensory organ development. J Clin Invest 2010; 120: 93–102.
Kenerson H, Dundon TA, Yeung RS . Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res 2005; 57: 67–75.
Knox S, Ge H, Dimitroff BD, Ren Y, Howe KA, Arsham AM et al. Mechanisms of TSC-mediated control of synapse assembly and axon guidance. PLoS One 2007; 2: e375.
Lee PS, Tsang SW, Moses MA, Trayes-Gibson Z, Hsiao LL, Jensen R et al. Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am J Respir Cell Mol Biol 2010; 42: 227–234.
Ma D, Bai X, Zou H, Lai Y, Jiang Y . Rheb GTPase controls apoptosis by regulating interaction of FKBP38 with Bcl-2 and Bcl-XL. J Biol Chem 2010; 285: 8621–8627.
Patch K, Stewart SR, Welch A, Ward RE . A second-site noncomplementation screen for modifiers of Rho1 signaling during imaginal disc morphogenesis in Drosophila. PLoS One 2009; 4: e7574.
Weisman R, Roitburg I, Schonbrun M, Harari R, Kupiec M . Opposite effects of tor1 and tor2 on nitrogen starvation responses in fission yeast. Genetics 2007; 175: 1153–1162.
Wilson C, Bonnet C, Guy C, Idziaszczyk S, Colley J, Humphreys V et al. Tsc1 haploinsufficiency without mammalian target of rapamycin activation is sufficient for renal cyst formation in Tsc1+/- mice. Cancer Res 2006; 66: 7934–7938.
Zhou X, Ikenoue T, Chen X, Li L, Inoki K, Guan KL . Rheb controls misfolded protein metabolism by inhibiting aggresome formation and autophagy. Proc Natl Acad Sci USA 2009; 106: 8923–8928.
Neuman NA, Henske EP . Non-canonical functions of the tuberous sclerosis complex-Rheb signalling axis. EMBO Mol Med 2011; 3: 189–200.
Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis 2012; 15: 33–45.
Govindarajan B, Willoughby L, Band H, Curatolo AS, Veledar E, Chen S et al. Cooperative benefit for the combination of rapamycin and imatinib in tuberous sclerosis complex neoplasia. Vascular Cell 2012; 4: 11.
Arbiser JL, Yeung R, Weiss SW, Arbiser ZK, Amin MB, Cohen C et al. The generation and characterization of a cell line derived from a sporadic renal angiomyolipoma: use of telomerase to obtain stable populations of cells from benign neoplasms. Am J Pathol 2001; 159: 483–491.
Arbiser JL, Govindarajan B, Bai X, Onda H, Kazlauskas A, Lim SD et al. Functional tyrosine kinase inhibitor profiling: a generally applicable method points to a novel role of platelet-derived growth factor receptor-beta in tuberous sclerosis. Am J Pathol 2002; 161: 781–786.
Pardal R, Ortega-Saenz P, Duran R, Lopez-Barneo J . Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 2007; 131: 364–377.
Lasser C, Eldh M, Lotvall J . Isolation and characterization of RNA-containing exosomes. J Vis Exp 2012; 59: e3037.
Tian T, Wang Y, Wang H, Zhu Z, Xiao Z . Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem 2010; 111: 488–496.
Acknowledgements
We thank Drs Maureen Murphy and Aristotelis Astreinidis for his invaluable comments. CPRIT grant RP 120168 to M Karbowniczek and the DoD grant BC 111038 to MM Markiewski.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Patel, B., Patel, J., Cho, JH. et al. Exosomes mediate the acquisition of the disease phenotypes by cells with normal genome in tuberous sclerosis complex. Oncogene 35, 3027–3036 (2016). https://doi.org/10.1038/onc.2015.358
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.358
This article is cited by
-
Neutral sphingomyelinase inhibition promotes local and network degeneration in vitro and in vivo
Cell Communication and Signaling (2023)
-
Notch transactivates Rheb to maintain the multipotency of TSC-null cells
Nature Communications (2017)
-
Tuberous sclerosis complex
Nature Reviews Disease Primers (2016)
-
Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake
Cellular and Molecular Neurobiology (2016)