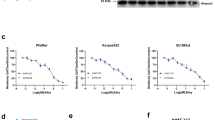
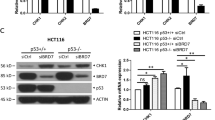
Abstract
Hec1 (highly expressed in cancer 1) or Nek2 (NIMA-related kinase 2) is often overexpressed in cancers with poor prognosis. Both are critical mitotic regulators, and phosphorylation of Hec1 S165 by Nek2 is required for proper chromosome segregation. Therefore, inactivation of Hec1 and Nek2 by targeting their interaction with small molecules represents an ideal strategy for tackling these types of cancers. Here we showed that new derivatives of INH (inhibitor for Nek2 and Hec1 binding) bind to Hec1 at amino acids 394–408 on W395, L399 and K400 residues, effectively blocking Hec1 phosphorylation on S165 by Nek2, and killing cancer cells at the nanomolar range. Mechanistically, the D-box (destruction-box) region of Nek2 specifically binds to Hec1 at amino acids 408–422, immediately adjacent to the INH binding motif. Subsequent binding of Nek2 to INH-bound Hec1 triggered proteasome-mediated Nek2 degradation, whereas the Hec1 binding defective Nek2 mutant, Nek2 R361L, resisted INH-induced Nek2 degradation. This finding unveils a novel drug-action mechanism where the binding of INHs to Hec1 forms a virtual death-trap to trigger Nek2 degradation and eventually cell death. Furthermore, analysis of the gene expression profiles of breast cancer patient samples revealed that co-elevated expressions of Hec1 and Nek2 correlated with the shortest survival. Treatment of mice with this kind of tumor with INHs significantly suppressed tumor growth without obvious toxicity. Taken together, the new INH derivatives are suitable for translation into clinical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Foley EA, Kapoor TM . Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 2013; 14: 25–37.
Walczak CE, Cai S, Khodjakov A . Mechanisms of chromosome behaviour during mitosis. Nat Rev Mol Cell Biol 2010; 11: 91–102.
Rath O, Kozielski F . Kinesins and cancer. Nat Rev Cancer 2012; 12: 527–539.
Chan KS, Koh CG, Li HY . Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis 2012; 3: e411.
Jordan M, Wilson L . Microtubules as a target for anticancer drugs. Nat Rev Cancer 2006; 4: 253–265.
Perez EA . Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther 2009; 8: 2086–2095.
Rowinsky E . The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 1997; 48: 353–374.
Sakowicz R, Finer JT, Beraud C, Crompton A, Lewis E, Fritsch A et al. Antitumor activity of a kinesin inhibitor. Cancer Res 2004; 64: 3276–3280.
Schmit TL, Ahmad N . Regulation of mitosis via mitotic kinases: new opportunities for cancer management. Mol Cancer Ther 2007; 6: 1920–1931.
Jackson JR, Patrick DR, Dar MM, Huang PS . Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer 2007; 7: 107–117.
Chen Y, Riley DJ, Chen PL, Lee WH . HEC, a novel nuclear protein rich in leucine heptad repeats specifically involved in mitosis. Mol Cell Biol 1997; 17: 6049–6056.
Sundin LJ, Guimaraes GJ, Deluca JG . The NDC80 complex proteins Nuf2 and Hec1 make distinct contributions to kinetochore-microtubule attachment in mitosis. Mol Biol Cell 2011; 22: 759–768.
Umbreit NT, Gestaut DR, Tien JF, Vollmar BS, Gonen T, Asbury CL et al. The Ndc80 kinetochore complex directly modulates microtubule dynamics. Proc Natl Acad Sci USA 2012; 109: 16113–16118.
Martin-Lluesma S, Stucke VM, Nigg EA . Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 2002; 297: 2267–2270.
Meraldi P, Draviam VM, Sorger PK . Timing and checkpoints in the regulation of mitotic progression. Dev Cell 2004; 7: 45–60.
Lin YT, Chen Y, Wu G, Lee WH . Hec1 sequentially recruits Zwint-1 and ZW10 to kinetochores for faithful chromosome segregation and spindle checkpoint control. Oncogene 2006; 25: 6901–6914.
Wei R, Ngo B, Wu G, Lee WH . Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol Biol Cell 2011; 22: 3584–3594.
DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED . Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 2006; 127: 969–982.
van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–536.
Glinsky GV, Berezovska O, Glinskii AB . Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest 2005; 115: 1503–1521.
Diaz-Rodriguez E, Sotillo R, Schvartzman JM, Benezra R . Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci USA 2008; 105: 16719–16724.
Li L, Yang L, Scudiero DA, Miller SA, Yu ZX, Stukenberg PT et al. Development of recombinant adeno-associated virus vectors carrying small interfering RNA (shHec1)-mediated depletion of kinetochore Hec1 protein in tumor cells. Gene Ther 2007; 14: 814–827.
Gurzov EN, Izquierdo M . RNA interference against Hec1 inhibits tumor growth in vivo. Gene Ther 2006; 13: 1–7.
Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH . Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem 2002; 277: 49408–49416.
Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J et al. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res 2008; 68: 8393–8399.
Qiu X.L, Li G, Wu G, Zhu J, Zhou L, Chen PL et al. Synthesis and biological evaluation of a series of novel inhibitor of Nek2/Hec1 analogues. J Med Chem 2009; 52: 1757–1767.
Fry AM, Meraldi P, Nigg EA . A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J 1998; 17: 470–481.
Fry AM . The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 2002; 21: 6184–6194.
Hayward DG, Fry AM . Nek2 kinase in chromosome instability and cancer. Cancer Lett 2006; 237: 155–166.
Vitale I, Galluzzi L, Castedo M, Kroemer G . Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol 2011; 12: 385–392.
Schmid I, Uittenbogaart CH, Giorgi JV . Sensitive method for measuring apoptosis and cell surface phenotype in human thymocytes by flow cytometry. Cytometry 1994; 15: 12–20.
Ngo B, Hu CM, Guo XE, Ngo B, Wei R, Zhu J et al. Complementary interhelical interactions between three buried Glu-Lys pairs within three heptad repeats are essential for Hec1-Nuf2 heterodimerization and mitotic progression. J Biol Chem 2013; 288: 34403–34413.
Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM . APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J 2011; 20: 7117–7127.
Hayward DG, Clarke RB, Faragher AJ, Pillai MR, Hagan IM, Fry AM . The centrosomal kinase Nek2 displays elevated levels of protein expression in human breast cancer. Cancer Res 2004; 64: 7370–7376.
Wang S, Li W, Liu N, Zhang F, Liu H, Liu F et al. Nek2A contributes to tumorigenic growth and possibly functions as potential therapeutic target for human breast cancer. J Cell Biochem 2012; 113: 1904–1914.
Bieche I, Vacher S, Lallemand F, Tozlu-Kara S, Bennani H, Beuzelin M et al. Expression analysis of mitotic spindle checkpoint genes in breast carcinoma: role of NDC80/HEC1 in early breast tumorigenicity, and a two-gene signature for aneuploidy. Mol Cancer 2011; 10: 23.
Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol 2004; 5: R80.
Hu J, Barbour LJ, Gokel GW . The indole side chain of tryptophan as a versatile pi-donor. J Am Chem Soc 2012; 124: 10940–10941.
Arkin MR, Wells JA . Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nat Rev Drug Discov 2004; 3: 301–317.
Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med 2004; 10: 1321–1328.
Shangary S, Wang S . Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol 2009; 49: 223–241.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004; 303: 844–848.
Zhu J, Zhou L, Wu G, Konig H, Lin X, Li G et al. A novel small molecule RAD51 inactivator overcomes imatinib-resistance in chronic myeloid leukaemia. EMBO Mol Med 2013; 5: 353–365.
Kokuryo T, Senga T, Yokoyama Y, Nagino M, Nimura Y, Hamaguchi M . Nek2 as an effective target for inhibition of tumorigenic growth and peritoneal dissemination of cholangiocarcinoma. Cancer Res 2007; 67: 9637–9642.
Barbagallo F, Paronetto MP, Franco R, Chieffi P, Dolci S, Fry AM et al. Increased expression and nuclear localization of the centrosomal kinase Nek2 in human testicular seminomas. J Pathol 2009; 217: 431–441.
Andreasson U, Dictor M, Jerkeman M, Berglund M, Sundström C, Linderoth J et al. Identification of molecular targets associated with transformed diffuse large B cell lymphoma using highly purified tumor cells. Am J Hematol 2009; 84: 803–808.
Zhou W, Yang Y, Xia J, Wang H, Salama ME, Xiong W et al. NEK2 induces drug resistance mainly through activation of efflux drug pumps and is associated with poor prognosis in myeloma and other cancers. Cancer cell 2013; 23: 48–62.
Cavasotto CN, Orry AJ, Abagyan RA . Structure-based identification of binding sites, native ligands and potential inhibitors for G-protein coupled receptors. Proteins 2003; 51: 423–433.
Acknowledgements
We thank the initial efforts by Guideng Li, Randy Wei and Yumay Chen on this project. This work was supported by an NIH grant (CA107568) to WHL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, CM., Zhu, J., Guo, X. et al. Novel small molecules disrupting Hec1/Nek2 interaction ablate tumor progression by triggering Nek2 degradation through a death-trap mechanism. Oncogene 34, 1220–1230 (2015). https://doi.org/10.1038/onc.2014.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.67
This article is cited by
-
Nek2 augments sorafenib resistance by regulating the ubiquitination and localization of β-catenin in hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2019)
-
Upregulation of FOXM1 leads to diminished drug sensitivity in myeloma
BMC Cancer (2018)
-
Overexpression of NIMA-related kinase 2 is associated with poor prognoses in malignant glioma
Journal of Neuro-Oncology (2017)
-
FOXM1 is a therapeutic target for high-risk multiple myeloma
Leukemia (2016)