Abstract
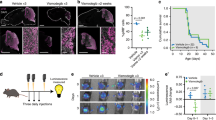
The identification of key tumorigenic events in Sonic Hedgehog (SHH) subgroup medulloblastomas (MBSHH) will be essential for the development of individualized therapies and improved outcomes. However, beyond confirmation of characteristic SHH pathway mutations, recent genome-wide sequencing studies have not revealed commonly mutated genes with widespread relevance as potential therapeutic targets. We therefore examined any role for epigenetic DNA methylation events in MBSHH using a cross-species approach to candidate identification, prioritization and validation. MBSHH-associated DNA methylation events were first identified in 216 subgrouped human medulloblastomas (50 MBSHH, 28 Wnt/Wingless, 44 Group 3 and 94 Group 4) and their conservation then assessed in tumors arising from four independent murine models of Shh medulloblastoma, alongside any role in tumorigenesis using functional assessments in mouse and human models. This strategy identified widespread regional CpG hypo-methylation of VAV1, leading to its elevated expression, as a conserved aberrant epigenetic event, which characterizes the majority of MBSHH tumors in both species, and is associated with a poor outcome in MBSHH patients. Moreover, direct modulation of VAV1 in mouse and human models revealed a critical role in tumor maintenance, and its abrogation markedly reduced medulloblastoma growth. Further, Vav1 activity regulated granule neuron precursor germinal zone exit and migration initiation in an ex vivo model of early postnatal cerebellar development. These findings establish VAV1 as a critical epigenetically regulated oncogene with a key role in MBSHH maintenance, and highlight its potential as a validated therapeutic target and prognostic biomarker for the improved therapy of medulloblastoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ et al. An animal model of MYC-driven medulloblastoma. Cancer Cell 2012; 21: 155–167.
Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathologica 2012; 123: 465–472.
Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C et al. A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell 2012; 21: 168–180.
Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010; 468: 1095–1099.
Pizer BL, Clifford SC . The potential impact of tumour biology on improved clinical practice for medulloblastoma: progress towards biologically driven clinical trials. Br J Neurosurg 2009; 23: 364–375.
Kool M, Jones DT, Jager N, Northcott PA, Pugh TJ, Hovestadt V et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell 2014; 25: 393–405.
Ng JM, Curran T . The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev 2011; 11: 493–501.
Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 2009; 361: 1173–1178.
Kimura H, Ng JM, Curran T . Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell 2008; 13: 249–260.
Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science (New York, NY) 2009; 326: 572–574.
Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ et al. Medulloblastomics: the end of the beginning. Nat Rev 2012; 12: 818–834.
Anderton JA, Lindsey JC, Lusher ME, Gilbertson RJ, Bailey S, Ellison DW et al. Global analysis of the medulloblastoma epigenome identifies disease-subgroup-specific inactivation of COL1A2. Neuro Oncol 2008; 10: 981–994.
Northcott PA, Nakahara Y, Wu X, Feuk L, Ellison DW, Croul S et al. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet 2009; 41: 465–472.
Hovestadt V, Remke M, Kool M, Pietsch T, Northcott PA, Fischer R et al. Robust molecular subgrouping and copy-number profiling of medulloblastoma from small amounts of archival tumour material using high-density DNA methylation arrays. Acta Neuropathologica 2013; 125: 913–916.
Schwalbe EC, Williamson D, Lindsey JC, Hamilton D, Ryan SL, Megahed H et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathologica 2013; 125: 359–371.
Uziel T, Zindy F, Xie S, Lee Y, Forget A, Magdaleno S et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev 2005; 19: 2656–2667.
Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene 2007; 26: 6442–6447.
Hatton BA, Villavicencio EH, Tsuchiya KD, Pritchard JI, Ditzler S, Pullar B et al. The Smo/Smo model: Hedgehog-induced medulloblastoma with 90% incidence and leptomeningeal spread. Cancer Res. 2008; 68: 1768–1776.
Goodrich LV, Milenkovic L, Higgins KM, Scott MP . Altered neural cell fates and medulloblastoma in mouse patched mutants. Science (New York, NY) 1997; 277: 1109–1113.
Lusher ME, Lindsey JC, Latif F, Pearson AD, Ellison DW, Clifford SC . Biallelic epigenetic inactivation of the RASSF1A tumor suppressor gene in medulloblastoma development. Cancer Res 2002; 62: 5906–5911.
Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol 2003; 21: 1581–1591.
Northcott PA, Shih DJ, Peacock J, Garzia L, Morrissy AS, Zichner T et al. Subgroup-specific structural variation across 1000 medulloblastoma genomes. Nature 2012; 488: 49–56.
Abe K, Whitehead IP, O'Bryan JP, Der CJ . Involvement of NH(2)-terminal sequences in the negative regulation of Vav signaling and transforming activity. J Biol Chem 1999; 274: 30410–30418.
Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R et al. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 2005; 7: 39–49.
Zindy F, Uziel T, Ayrault O, Calabrese C, Valentine M, Rehg JE et al. Genetic alterations in mouse medulloblastomas and generation of tumors de novo from primary cerebellar granule neuron precursors. Cancer Res 2007; 67: 2676–2684.
Hatten ME, Roussel MF . Development and cancer of the cerebellum. Trends Neurosci 2011; 34: 134–142.
Famulski JK, Trivedi N, Howell D, Yang Y, Tong Y, Gilbertson R et al. Siah regulation of Pard3A controls neuronal cell adhesion during germinal zone exit. Science (New York, NY) 2010; 330: 1834–1838.
Katzav S, Cleveland JL, Heslop HE, Pulido D . Loss of the amino-terminal helix-loop-helix domain of the vav proto-oncogene activates its transforming potential. Mol Cell Biol 1991; 11: 1912–1920.
Lindsey JC, Schwalbe EC, Potluri S, Bailey S, Williamson D, Clifford SC . TERT promoter mutation and aberrant hypermethylation are associated with elevated expression in medulloblastoma and characterise the majority of non-infant SHH subgroup tumours. Acta Neuropathologica 2014; 127: 307–309.
Remke M, Ramaswamy V, Peacock J, Shih DJ, Koelsche C, Northcott PA et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathologica 2013; 126: 917–929.
Denkinger DJ, Borges CR, Butler CL, Cushman AM, Kawahara RS . Genomic organization and regulation of the vav proto-oncogene. Biochimica et Biophysica Acta 2000; 1491: 253–262.
Katzav S . Flesh and blood: the story of Vav1, a gene that signals in hematopoietic cells but can be transforming in human malignancies. Cancer Lett 2007; 255: 241–254.
Wu X, Northcott PA, Dubuc A, Dupuy AJ, Shih DJ, Witt H et al. Clonal selection drives genetic divergence of metastatic medulloblastoma. Nature 2012; 482: 529–533.
Wechsler-Reya RJ, Scott MP . Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 1999; 22: 103–114.
Hahn H, Wojnowski L, Specht K, Kappler R, Calzada-Wack J, Potter D et al. Patched target Igf2 is indispensable for the formation of medulloblastoma and rhabdomyosarcoma. J Biol Chem 2000; 275: 28341–28344.
Roussel MF, Hatten ME . Cerebellum development and medulloblastoma. Curr Topics Dev Biol 2011; 94: 235–282.
Quevedo C, Sauzeau V, Menacho-Marquez M, Castro-Castro A, Bustelo XR . Vav3-deficient mice exhibit a transient delay in cerebellar development. Mol Biol Cell 2010; 21: 1125–1139.
Fischer KD, Kong YY, Nishina H, Tedford K, Marengere LE, Kozieradzki I et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol 1998; 8: 554–562.
Sebban S, Farago M, Gashai D, Ilan L, Pikarsky E, Ben-Porath I et al. Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PLoS ONE 2013; 8: e54321.
Wells CM, Bhavsar PJ, Evans IR, Vigorito E, Turner M, Tybulewicz V et al. Vav1 and Vav2 play different roles in macrophage migration and cytoskeletal organization. Exp Cell Res 2005; 310: 303–310.
Lazer G, Katzav S . Guanine nucleotide exchange factors for RhoGTPases: good therapeutic targets for cancer therapy? Cell Signal 2011; 23: 969–979.
Oberley MJ, Wang DS, Yang DT . Vav1 in hematologic neoplasms, a mini review. Am J Blood Res 2012; 2: 1–8.
Ladd-Acosta C, Pevsner J, Sabunciyan S, Yolken RH, Webster MJ, Dinkins T et al. DNA methylation signatures within the human brain. Am J Hum Genet 2007; 81: 1304–1315.
Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res 2006; 16: 383–393.
Schwalbe EC, Lindsey JC, Straughton D, Hogg TL, Cole M, Megahed H et al. Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res 2011; 17: 1883–1894.
Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL et al. Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol 2011; 29: 1400–1407.
Langdon JA, Lamont JM, Scott DK, Dyer S, Prebble E, Bown N et al. Combined genome-wide allelotyping and copy number analysis identify frequent genetic losses without copy number reduction in medulloblastoma. Genes Chromosomes Cancer 2006; 45: 47–60.
Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J . Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res 2006; 66: 248–258.
Zhao H, Ayrault O, Zindy F, Kim JH, Roussel MF . Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes Dev 2008; 22: 722–727.
RDevelopmentCoreTeam. R: a language and environment for statistical computing. In R Foundation for Statistical Computing, Vienna 2011.
Acknowledgements
This work was supported by grants from the Brain Tumour Charity (grants 16/46 (SCC and SB) and 16/92 (SCC, SB, DWE and JCL)), NIH CA-096832, CA-02165-29 (MFR), the American Lebanese Syrian Associated Charities of St Jude Children’s Research Hospital (MFR, DK), the Mochida Foundation (DK), the Anderson fellowship (DK), LoveOliver (SCC and DW) and Cancer Research UK (grant C8464/A13457; SCC, SB and DWE). Cell lines D425Med and MEB-Med8A were gifts from Dr D Bigner (Duke University, Durham, USA) and Professor T Pietsch (University of Bonn Medical Centre, Bonn, Germany), respectively. Four normal cerebellar DNAs were gifts from Dr M Fruhwald (University of Munster, Munster, Germany). Vav1CA cDNA was a gift from Dr S Katzav (Hebrew University, Jerusalem, Israel). DAOY and D283Med cell lines were from the ATCC (Manassas, VA, USA). Approval from the Newcastle and North Tyneside Research Ethics Committee (study reference 07/Q0905/71) was obtained for the collection, storage, and biological study of all material described. Medulloblastomas investigated in this study include samples provided by the UK Children’s Cancer and Leukaemia Group (CCLG) as part of CCLG-approved biological study BS-2007-04.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lindsey, J., Kawauchi, D., Schwalbe, E. et al. Cross-species epigenetics identifies a critical role for VAV1 in SHH subgroup medulloblastoma maintenance. Oncogene 34, 4746–4757 (2015). https://doi.org/10.1038/onc.2014.405
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.405