Abstract
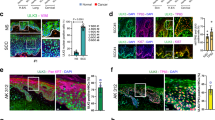
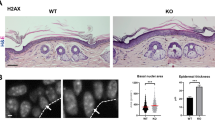
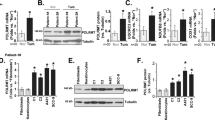
The protein deacetylase SIRT1 regulates various pathways in metabolism, aging and cancer. However, the role of SIRT1 in skin cancer remains unclear. Here, using mice with targeted deletions of SIRT1 in their epidermis in both resistant B6 and sensitive SKH1 hairless backgrounds, we show that the role of SIRT1 in skin cancer development induced by ultraviolet B (UVB) radiation is dependent on its gene dose. Keratinocyte-specific heterozygous deletion of SIRT1 promotes UVB-induced skin tumorigenesis, whereas homozygous deletion of SIRT1 suppresses skin tumor development but sensitizes the B6 mice to chronic solar injury. In mouse skin, SIRT1 is haploinsufficient for UVB-induced DNA damage repair and expression of xeroderma pigmentosum C (XPC), a protein critical for repairing UVB-induced DNA damage. As compared with normal human skin, downregulation of SIRT1 is in parallel with downregulation of XPC in human cutaneous squamous cell carcinoma at both the protein and mRNA levels. In contrast, homozygous SIRT1 deletion in mouse skin augments p53 acetylation and expression of its transcriptional target Noxa, and sensitizes the epidermis to UVB-induced apoptosis in vivo, while heterozygous SIRT1 deletion has no such effect. The gene dosage-dependent function of SIRT1 in DNA repair and cell survival is consistent with the dual roles of SIRT1 in UVB-induced skin tumorigenesis. Our results reveal the gene dosage-dependent in vivo functions of SIRT1 in skin tumorigenesis and may shed light on the role of SIRT1 in epithelial cancer induced by DNA damage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Abbreviations
- cHet:
-
keratinocyte-specific heterozygous SIRT1 deletion
- cKO:
-
keratinocyte-specific homozygous SIRT1 deletion
- NHEK:
-
normal human epidermal keratinocytes
- PAP:
-
papilloma
- SCC:
-
squamous cell carcinoma
- SIRT1:
-
sirtuin 1
- UVB:
-
ultraviolet B
- WT:
-
wild-type
- XP:
-
xeroderma pigmentosum
- XPC:
-
xeroderma pigmentosum group C.
References
Haigis MC, Guarente LP . Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev 2006; 20: 2913–2921.
Blander G, Guarente L . The Sir2 family of protein deacetylases. Annu Rev Biochem 2004; 73: 417–435.
Michan S, Sinclair D . Sirtuins in mammals: insights into their biological function. Biochem J 2007; 404: 1–13.
Haigis MC, Sinclair DA . Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol 2010; 5: 253–295.
Brooks CL, Gu W . How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer 2009; 9: 123–128.
Deng CX . SIRT1, is it a tumor promoter or tumor suppressor? Int J Biol Sci 2009; 5: 147–152.
Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 2001; 107: 137–148.
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 2001; 107: 149–159.
Prives C, Manley JL . Why is p53 acetylated? Cell 2001; 107: 815–818.
Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res 2007; 67: 6612–6618.
Bradbury CA, Khanim FL, Hayden R, Bunce CM, White DA, Drayson MT et al. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia 2005; 19: 1751–1759.
Stunkel W, Peh BK, Tan YC, Nayagam VM, Wang X, Salto-Tellez M et al. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J 2007; 2: 1360–1368.
Hida Y, Kubo Y, Murao K, Arase S . Strong expression of a longevity-related protein, SIRT1, in Bowen's disease. Arch Dermatol Res 2007; 299: 103–106.
Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell 2008; 32: 11–20.
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003; 425: 191–196.
Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 2004; 13: 627–638.
Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004; 305: 390–392.
Wang RH, Sengupta K, Li C, Kim HS, Cao L, Xiao C et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 2008; 14: 312–323.
Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One 2008; 3: e2020.
Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK et al. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 2008; 135: 907–918.
Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O et al. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun 2010; 1: 3.
Powell MJ, Casimiro MC, Cordon-Cardo C, He X, Yeow WS, Wang C et al. Disruption of a Sirt1-dependent autophagy checkpoint in the prostate results in prostatic intraepithelial neoplasia lesion formation. Cancer Res 2011; 71: 964–975.
Herranz D, Serrano M . SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010; 10: 819–823.
Herranz D, Maraver A, Canamero M, Gomez-Lopez G, Inglada-Perez L, Robledo M et al. SIRT1 promotes thyroid carcinogenesis driven by PTEN deficiency. Oncogene 2013; 32: 4052–4056.
Yuan Z, Seto E . A functional link between SIRT1 deacetylase and NBS1 in DNA damage response. Cell Cycle 2007; 6: 2869–2871.
Fan W, Luo J . SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Molecular cell 2010; 39: 247–258.
Ming M, Shea CR, Guo X, Li X, Soltani K, Han W et al. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci USA 2010; 107: 22623–22628.
Smith J . Human Sir2 and the 'silencing' of p53 activity. Trends Cell Biol 2002; 12: 404–406.
Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA 2003; 100: 10794–10799.
Kamel C, Abrol M, Jardine K, He X, McBurney MW . SirT1 fails to affect p53-mediated biological functions. Aging Cell 2006; 5: 81–88.
Ming M, Shea CR, Guo X, Li X, Soltani K, Han W et al. Regulation of global genome nucleotide excision repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci USA 107: 22623–22628.
Fan W, Luo J . SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Mol Cell 39: 247–258.
Jeong J, Juhn K, Lee H, Kim SH, Min BH, Lee KM et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med 2007; 39: 8–13.
Yuan Z, Zhang X, Sengupta N, Lane WS, Seto E . SIRT1 regulates the function of the Nijmegen breakage syndrome protein. Mol Cell 2007; 27: 149–162.
Sugasawa K . Xeroderma pigmentosum genes: functions inside and outside DNA repair. Carcinogenesis 2008; 29: 455–465.
Berg RJ, Ruven HJ, Sands AT, de Gruijl FR, Mullenders LH . Defective global genome repair in XPC mice is associated with skin cancer susceptibility but not with sensitivity to UVB induced erythema and edema. J Invest Dermatol 1998; 110: 405–409.
Cheo DL, Meira LB, Burns DK, Reis AM, Issac T, Friedberg EC . Ultraviolet B radiation-induced skin cancer in mice defective in the Xpc, Trp53, and Apex (HAP1) genes: genotype-specific effects on cancer predisposition and pathology of tumors. Cancer Res 2000; 60: 1580–1584.
Vousden KH, Lane DP . p53 in health and disease. Nat Rev Mol Cell Biol 2007; 8: 275–283.
Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol 2006; 26: 28–38.
Boily G, He XH, Pearce B, Jardine K, McBurney MW . SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene 2009; 28: 2882–2893.
McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol 2003; 23: 38–54.
Itoh T, Cado D, Kamide R, Linn S . DDB2 gene disruption leads to skin tumors and resistance to apoptosis after exposure to ultraviolet light but not a chemical carcinogen. Proc Natl Acad Sci USA 2004; 101: 2052–2057.
Oka M, Edamatsu H, Kunisada M, Hu L, Takenaka N, Dien S et al. Enhancement of ultraviolet B-induced skin tumor development in phospholipase Cepsilon-knockout mice is associated with decreased cell death. Carcinogenesis 2010; 31: 1897–1902.
Li H, Rajendran GK, Liu N, Ware C, Rubin BP, Gu Y . SirT1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res 2007; 9: R1.
Wu CL, Qiang L, Han W, Ming M, Viollet B, He YY . Role of AMPK in UVB-induced DNA damage repair and growth control. Oncogene 2013; 32: 2682–2689.
Acknowledgements
This work was supported by the NIH/NIEHS Grant ES016936 (to YYH), the American Cancer Society (ACS) Grant RSG-13-078-01 (to YYH), the University of Chicago Cancer Research Center (P30 CA014599), the CTSA (NIH UL1RR024999) and the University of Chicago Friends of Dermatology Endowment Fund. We thank Terri Li for Ki67 and TUNEL and XPC immunohistochemical analysis, and Dr Xiaobing Shi for kindly providing the WT and K382R mutant p53 plasmids.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
KS serves on the board of directors in Elorac Pharma and receives stocks from Elorac Pharma, DUSA, Winston and Gideon Pharmaceuticals, but none of them are relevant to this study. All the other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ming, M., Soltani, K., Shea, C. et al. Dual role of SIRT1 in UVB-induced skin tumorigenesis. Oncogene 34, 357–363 (2015). https://doi.org/10.1038/onc.2013.583
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.583
Keywords
This article is cited by
-
Sirtuin1 (SIRT1) is involved in the anticancer effect of black raspberry anthocyanins in colorectal cancer
European Journal of Nutrition (2023)
-
miR-29a-SIRT1-Wnt/β-Catenin Axis Regulates Tumor Progression and Survival in Hepatocellular Carcinoma
Biochemical Genetics (2023)
-
Epidermal SIRT1 regulates inflammation, cell migration, and wound healing
Scientific Reports (2017)