Abstract
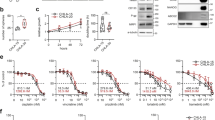
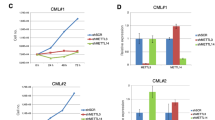
When mTOR inhibitor rapalogs prevent cap-dependent translation of cell-cycle proteins like c-myc, continuing tumor cell growth depends on cap-independent translation, which is mediated by internal ribosome entry sites (IRESes) located in the 5′-UTR (untranslated region) of transcripts. To investigate if rapalog-induced activation of MNK kinases had a role in such IRES activity, we studied multiple myeloma (MM) cells. Rapamycin (RAP)-activated MNK1 kinase activity in MM cell lines and primary specimens by a mitogen-activated protein kinase-dependent mechanism. Pharmacological inhibition of MNK activity or genetic silencing of MNK1 prevented a rapalog-induced upregulation of c-myc IRES activity. Although RAP, used alone, had little effect on myc protein expression, when combined with a MNK inhibitor, myc protein expression was abrogated. In contrast, there was no inhibition of myc RNA, consistent with an effect on myc translation. In a RAP-resistant MM cell lines as well as a resistant primary MM specimen, co-exposure to a MNK inhibitor or MNK1 knockdown significantly sensitized cells for RAP-induced cytoreduction. Studies in MNK-null murine embryonic fibroblasts additionally supported a role for MNK kinases in RAP-induced myc IRES stimulation. These results indicate that MNK kinase activity has a critical role in the fail-safe mechanism of IRES-dependent translation when mTOR is inhibited. As kinase activity also regulated sensitivity to RAP, the data also provide a rationale for therapeutically targeting MNK kinases for combined treatment with mTOR inhibitors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
25 August 2023
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1038/s41388-023-02818-z
References
Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Peterson R et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci 2001; 98: 10314–10319.
Gera J, Mellinghoff I, Shi Y, Rettig M, Tran C, Hsu JH et al. AKT activity determines sensitivity to mTOR inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem 2004; 279: 2737–2746.
Frost P, Moatomed F, Hoang B, Shi Y, Gera J, Yan H et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood 2004; 104: 4181–4187.
Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res 2002; 62: 5027–5034.
Shi Y, Sharma A, Wu H, Lichtenstein A, Gera J . Cyclin D1 and c-myc IRES-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J Biol Chem 2005; 280: 10964–109973.
Frost P, Shi Y, Hoang B, Gera J, Lichtenstein A . Regulation of D-cyclin translation inhibition in myeloma cells treated with mTOR inhibitors: rationale for combined treatment with ERK inhibitors and rapamycin. Mol Cancer Ther 2009; 8: 83–93.
Subkhankulova T, Mitchell SA, Willis AE . IRES-mediated initiation of c-myc protein synthesis following genotoxic stress. Biochem J 2001; 359: 183–192.
Stoneley M, Chappell SA, Jopling CL, Dickens M, MacFarlane M, Willis AE . C-myc protein synthesis is initiated from the IRES during apoptosis. Mol Cell Biol 2000; 20: 1162–1169.
Parra JL, Buxade M, Proud CG . Features of the catalytic domains and C termini of the MAPK signal-integrating kinases MNK1 and MNK2 determine their differing activities and regulatory properties. J Biol Chem 2005; 280: 37623–37633.
Wang X, Yue P, Chan C-B, Ye K, Ueda T, Watanabe-Fukunaga R et al. Inhibition of mTOR induces PI3-kinase-dependent and MNK-mediated eIF-4E phosphorylation. Mol Cell Biol 2007; 27: 7405–7413.
Ross G, Dyer JR, Castellucci VF, Sossin WS . MNK is a negative regulator of cap-dependent translation in Aplysia neurons. J Neurochem 2006; 97: 79–91.
Buxade M, Parra JL, Rousseau S, Shpiro N, Marquez R, Morrice N et al. The MNKs are novel components in the control of TNF alpha biosynthesis andphosphorylate and regulate hnRNP A1. Immunity 2005; 23: 177–189.
Scheper GC, Morrice NA, Kleijn M, Proud CG . The mitogen-activated protein kinase signal-integrating kinase MNK2 is an eIF-4E kinase with high levels of basal activity in mammalian cells. Mol Cell Biol 2001; 21: 743–754.
Scheper GC, Parra J-L, Wilson ML, van Kollenburg B, Vertegaal ACO, Han ZG et al. The N and C termini of the splice variants of the human MNK2 determine activity and localization. Mol Cell Biol 2003; 23: 5692–5705.
Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R . MNK2 and MNK1 are essential for constitutive and inducible phosphorylation of eIF-4E but not for cell growth or development. Mol Cell Biol 2004; 24: 6539–6549.
Parra-Palau JL, Scheper GC, Wilson ML, Proud CG . Features in the N and C termini of the MAPK-integrating kinase MNK1 mediate its nucleocytoplasmic shuttling. J Biol Chem 2003; 278: 44197–44204.
Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N . Human eIF4G recruits MNK1 to phosphorylate eIF-4E. EMBO J 1999; 18: 270–279.
Chrestensen CA, Shuman JK, Eschenroeder A, Worthington M, Gram H, Sturgill TW . MNK1 and MNK2 regulation in HER2-overexpressing breast cancer lines. J Biol Chem 2007; 282: 4243–4252.
Stoneley M, Willis AE . Cellular IRESes: structures, trans-acting factors and regulation of gene expression. Oncogene 2004; 23: 3200–3207.
Paulin FEM, West MJ, Sullivan NF, Whitney RL, Lyne L, Willis AE . Aberrant translational control of the c-myc gene in multiple myeloma. Oncogene 1996; 13: 505–513.
Chappell SA, LeQuesne JPC, Paulin FEM, deSchoolmeester ML, Stoneley M, Soutar RL et al. A mutation in the c-myc IRES leads to enhanced internal ribosome entry in multiple myeloma: a novel mechanism of oncogene de-regulation. Oncogene 2000; 19: 4437–4440.
Sun S-Y, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H et al. Activation of AKT and eIF4E survival pathways by rapamycin-mediated mTOR inhibition. Cancer Res 2005; 65: 7052–7058.
Shi Y, Frost PJ, Hoang B, Benavides A, Sharma S, Gera J et al. IL-6-induced stimulation of c-myc translation in multiple myeloma cells is mediated by myc IRES function and the RNA-binding protein hnRNP A1. Cancer Res 2008; 68: 10215–10222.
Jo OD, Martin J, Bernath A, Masri J, Lichtenstein A, Gera J . Heterogeneous nuclear ribonucleoprotein A'1 regulates cyclin D and c-myc IRES function through AKT signaling. J Biol Chem 2008; 283: 23274–23287.
Zhu Y, Sun Y, Mao O, Jin KL, Greenberg D . Expression of poly(C) binding proteins is differentially regulated by hypoxia and ischemia in cortical neurons. Neuroscience 2002; 110: 191–198.
Evans JR, Mitchell SA, Spriggs KA, Ostrowski J, Bomsztyk K, Ostarek D et al. Members of the poly (rC) binding protein family stimulate the activity of the c-myc IRES in vitro and in vivo. Oncogene 2003; 22: 8012–8020.
Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 2009; 137: 1–14.
Farag SS, Zhang S, Jansak BS, Wang X, Kraut E, Chan K et al. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leuk Res 2009; 33: 1475–1480.
Ogata A, Chauhan D, Teoh G, Treon S, Urashima M, Schlossman RL et al. IL-6 triggers cell growth via the RAS-dependent mitogen-activated protein kinase cascade. J Immunol 1997; 159: 2212–2221.
Paquette RL, Berenson J, Lichtenstein A, McCormick F, Koeffler HP . RAS mutations in multiple myeloma. Oncogene 1990; 5: 1569–1663.
Hoang B, Frost P, Shi Y, Belanger E, Benavides A, Pezeshkpour G et al. Targeting TORC2 in multiple myeloma with a new mTOR kinase inhibitor. Blood 2010; 116: 4560–4568.
Waskiewicz AJ, Flynn A, Proud CG, Cooper JA . Mitogen-activated protein kinases activate the serine/threonine kinses MNK1 and MNK2. EMBO J 1997; 16: 1909–1920.
Acknowledgements
We thank the UCLA Jonsson Comprehensive Cancer Center vector core lab for assistance in generating the lentiviral shRNA vectors. This work was supported by research funds of the Veteran's Administration, the Multiple Myeloma Research Foundation and the Department of Defense and NIH Grants RO1 CA109312 and RO1 CA111448.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Shi, Y., Frost, P., Hoang, B. et al. MNK kinases facilitate c-myc IRES activity in rapamycin-treated multiple myeloma cells. Oncogene 32, 190–197 (2013). https://doi.org/10.1038/onc.2012.43
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.43
Keywords
This article is cited by
-
Paired miRNA- and messenger RNA-sequencing identifies novel miRNA-mRNA interactions in multiple myeloma
Scientific Reports (2022)
-
Translational control of PML contributes to TNFα-induced apoptosis of MCF7 breast cancer cells and decreased angiogenesis in HUVECs
Cell Death & Differentiation (2016)
-
RETRACTED ARTICLE: Therapeutic potential of targeting IRES-dependent c-myc translation in multiple myeloma cells during ER stress
Oncogene (2016)
-
MNKs act as a regulatory switch for eIF4E1 and eIF4E3 driven mRNA translation in DLBCL
Nature Communications (2014)
-
miR-137 regulates the constitutive androstane receptor and modulates doxorubicin sensitivity in parental and doxorubicin-resistant neuroblastoma cells
Oncogene (2014)