Abstract
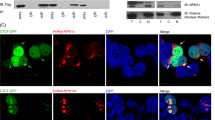
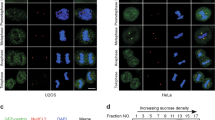
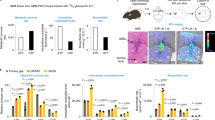
Nucleophosmin (NPM), a ubiquitously and abundantly expressed protein, occurs in the nucleolus, shuttling between the nucleoplasm and cytoplasm. The NPM gene is mutated in almost 30% of human acute myeloid leukemia cells. NPM interacts with p53 and p19Arf, directs localization of p19Arf in the nucleolus and protects the latter from degradation. Hepatocyte odd protein shuttling (HOPS) is also a ubiquitously expressed protein that moves between the nucleus and cytoplasm. Within the nucleus of resting cells, HOPS overexpression causes cell cycle arrest in G0/G1. HOPS knockdown causes centrosome hyperamplification leading to multinucleated cells and the formation of micronuclei. We demonstrate a direct interaction of HOPS with NPM and p19Arf, resulting in a functionally active trimeric complex. NPM appeared to regulate HOPS half-life, which, in turn, stabilized p19Arf and controlled its localization in the nucleolus. These findings suggest that HOPS acts as a functional bridge in the interaction between NPM and p19Arf, providing new mechanistic insight into how NPM and p19Arf will oppose tumor cell proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Colombo E, Alcalay M, Pelicci PG . Nucleophosmin and its complex network: a possible therapeutic target in hematological diseases. Oncogene 2011; 30: 2595–2609.
Borer RA, Lehner CF, Eppenberger HM, Nigg EA . Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989; 56: 379–390.
Szebeni A, Olson MO . Nucleolar protein B23 has molecular chaperone activities. Protein Sci 1999; 8: 905–912.
Colombo E, Bonetti P, Lazzerini Denchi E, Martinelli P, Zamponi R et al. Nucleophosmin is required for DNA integrity and p19Arf protein stability. Mol Cell Biol 2005; 25: 8874–8886.
Grisendi S, Bernardi R, Rossi M, Cheng K, Khandker L, Manova K et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 2005; 437: 147–153.
Colombo E, Martinelli P, Zamponi R, Shing DC, Bonetti P, Luzi L et al. Delocalization and destabilization of the Arf tumor suppressor by the leukemia-associated NPM mutant. Cancer Res 2006; 66: 3044–3050.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Acute Leukemia Working Party. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266.
Alcalay M, Tiacci E, Bergomas R, Bigerna B, Venturini E, Minardi SP et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood 2005; 106: 899–902.
Falini B, Bolli N, Shan J, Martelli MP, Liso A, Pucciarini A et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood 2006; 107: 4514–4523.
Grisendi S, Mecucci C, Falini B, Pandolfi PP . Nucleophosmin and cancer. Nat Rev Cancer 2006; 6: 493–505.
Colombo E, Marine JC, Danovi D, Falini B, Pelicci PG . Nucleophosmin regulates the stability and transcriptional activity of p53. Nat Cell Biol 2002; 4: 529–533.
Itahana K, Bhat KP, Jin A, Itahana Y, Hawke D, Kobayashi R et al. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol Cell 2003; 12: 1151–1164.
Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 2005; 434: 864–870.
Korgaonkar C, Hagen J, Tompkins V, Frazier AA, Allamargot C, Quelle FW et al. Nucleophosmin (B23) targets ARF to nucleoli and inhibits its function. Mol Cell Biol 2005; 25: 1258–1271.
Della Fazia MA, Castelli M, Bartoli D, Pieroni S, Pettirossi V, Piobbico D et al. HOPS: a novel cAMP-dependent shuttling protein involved in protein synthesis regulation. J Cell Sci 2005; 118: 3185–3194.
Pieroni S, Della Fazia MA, Castelli M, Piobbico D, Bartoli D, Brunacci C et al. HOPS is an essential constituent of centrosome assembly. Cell Cycle 2008; 7: 1462–1466.
Yang H, Takagi H, Konishi Y, Ageta H, Ikegami K, Yao I et al. Transmembrane and ubiquitin-like domain-containing protein 1 (Tmub1/HOPS) facilitates surface expression of GluR2-containing AMPA receptors. PLoS One 2008; 3: e2809.
Zhang W, Savelieva KV, Suwanichkul A, Small DL, Kirkpatrick LL, Xu N et al. Transmembrane and ubiquitin-like domain containing 1 (Tmub1) regulates locomotor activity and wakefulness in mice and interacts with CAMLG. PLoS One 2010; 5: e11261.
Wang W, Budhu A, Forgues M, Wang XW . Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat Cell Biol 2005; 7: 823–830.
Shinmura K, Tarapore P, Tokuyama Y, George KR, Fukasawa K . Characterization of centrosomal association of nucleophosmin/B23 linked to Crm1 activity. FEBS Lett 2005; 579: 6621–6634.
Bertwistle D, Sugimoto M, Sherr CJ . Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol Cell Biol 2004; 24: 985–996.
Enomoto T, Lindström MS, Jin A, Ke H, Zhang Y . Essential role of the B23/NPM core domain in regulating ARF binding and B23 stability. J Biol Chem 2006; 281: 18463–18472.
Gjerset RA, Bandyopadhyay K . Regulation of p14ARF through subnuclear compartmentalization. Cell Cycle 2006; 5: 686–690.
Moulin S, Llanos S, Kim SH, Peters G . Binding to nucleophosmin determines the localization of human and chicken ARF but not its impact on p53. Oncogene 2008; 27: 2382–2389.
Serrano M . The INK4a/ARF locus in murine tumorigenesis. Carcinogenesis 2000; 21: 865–869.
Sherr CJ . The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol 2001; 2: 731–737.
Cazzaniga G, Dell’Oro MG, Mecucci C, Giarin E, Masetti R, Rossi V et al. Nucleophosmin mutations in childhood acute myelogenous leukemia with normal karyotype. Blood 2005; 106: 1419–1422.
Chou WC, Tang JL, Lin LI, Yao M, Tsay W, Chen CY et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res 2006; 66: 3310–3316.
Pasqualucci L, Liso A, Martelli MP, Bolli N, Pacini R, Tabarrini A et al. Mutated nucleophosmin detects clonal multilineage involvement in acute myeloid leukemia: impact on WHO classification. Blood 2006; 108: 4146–4155.
Braoudaki M, Papathanassiou C, Katsibardi K, Tourkadoni N, Karamolegou K, Tzortzatou-Stathopoulou F . The frequency of NPM1 mutations in childhood acute myeloid leukemia. J Hematol Oncol 2010; 3: 41.
den Besten W, Kuo ML, Williams RT, Sherr CJ . Myeloid leukemia associated nucleophosmin mutants perturb p53-dependent and independent activities of the Arf tumor suppressor protein. Cell Cycle 2005; 4: 1593–1598.
Sherr CJ, Weber JD . The ARF/p53 pathway. Curr Opin Genet Dev 2000; 10: 94–99.
Manfredi JJ . The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes Dev 2010; 24: 1580–1589.
Campisi J . Aging and cancer cell biology. Aging Cell 2007; 6: 261–263.
Coutts AS, La Thangue NB . Mdm2 widens its repertoire. Cell Cycle 2007; 6: 827–829.
Sherr CJ . Tumor surveillance via the ARF-p53 pathway. Genes Dev 1998; 12: 2984–2991.
Cordell JL, Pulford KA, Bigerna B, Roncador G, Banham A, Colombo E et al. Detection of normal and chimeric nucleophosmin in human cells. Blood 1999; 93: 632–642.
Brunacci C, Piobbico D, Bartoli D, Castelli M, Pieroni S, Bellet MM et al. Identification and characterization of a novel peptide interacting with cAMP-responsive elements binding and cAMP-responsive elements modulator in mouse liver. Liver Int 2010; 30: 388–395.
Acknowledgements
We thank Paolo Puccetti and Stefano Brancorsini for the critical reading of the manuscript, Silvano Pagnotta for technical assistance. This study was supported by grants from the Associazione Umbra Contro il Cancro (AUCC), Fondazione Guido Berlucchi, the Associazione Italiana Ricerca sul Cancro (AIRC), Only the Brave Foundation and Fondazione Cassa di Risparmio di Perugia. GS is recipient of a PRIN Project n. 2008BH7KA2_003. DB, CB and DP are recipient of the AUCC fellowship program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Castelli, M., Pieroni, S., Brunacci, C. et al. Hepatocyte odd protein shuttling (HOPS) is a bridging protein in the nucleophosmin-p19Arf network. Oncogene 32, 3350–3358 (2013). https://doi.org/10.1038/onc.2012.353
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.353
Keywords
This article is cited by
-
Promoting anti-tumor immunity by targeting TMUB1 to modulate PD-L1 polyubiquitination and glycosylation
Nature Communications (2022)
-
HOPS/Tmub1 involvement in the NF-kB-mediated inflammatory response through the modulation of TRAF6
Cell Death & Disease (2020)
-
TMUB1 Inhibits BRL-3A Hepatocyte Proliferation by Interfering with the Binding of CAML to Cyclophilin B through its TM1 Hydrophobic Domain
Scientific Reports (2018)