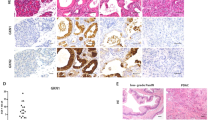
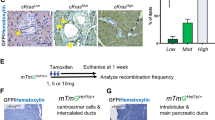
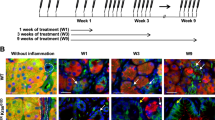
Abstract
Pancreatic carcinoma, a leading cause of cancer death, is thought to develop out of pancreatic intraepithelial neoplasia (PanIN). PanIN lesions have not yet attained the fully malignant phenotype, but show increased proliferation and dysplasia, and frequently bear an oncogenic KRAS mutation. Pancreatic cancer development is associated with increased activity of the transcription factor NF-κB. NEMO (IKKγ) is a subunit of the IKK complex essential for the activation of canonical NF-κB signaling and has been ascribed both oncogenic and tumor-suppressive roles in gastrointestinal tumors. Here, we wanted to address the function of NEMO in pancreatic tumorigenesis. We therefore conditionally ablated NEMO in a mouse model for pancreatic carcinoma based on the expression of oncogenic KRAS in pancreatic precursor cells. Mice were analyzed for PanIN lesions and for the activation of associated signaling pathways. NEMO ablation in the pancreas, while in itself not causing any overt pathology, led to a drastic (>93%) decrease in the prevalence of both low-grade and high-grade PanIN in 10-month-old mice expressing oncogenic KRAS. Also, the inflammatory and fibrotic response associated with KRAS action in the pancreas was virtually abolished, including expression of inflammatory cytokines and activation of the interleukin-6/STAT3 axis. Moreover, the activation of MAPK signaling, Notch and KLF4 signaling normally observed in KRAS-induced PanIN was strongly reduced or absent when NEMO was ablated. Our study suggests that NEMO, an IKK subunit necessary for canonical NF-κB activation, is dispensable for normal pancreatic development and function, but essential for the propagation of KRAS-induced PanIN lesions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schneider G, Siveke JT, Eckel F, Schmid RM . Pancreatic cancer: basic and clinical aspects. Gastroenterology 2005; 128: 1606–1625.
Hidalgo M . Pancreatic cancer. N Engl J Med 2010; 362: 1605–1617.
Hruban RH, Maitra A, Goggins M . Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008; 1: 306–316.
Lohr M, Kloppel G, Maisonneuve P, Lowenfels AB, Luttges J . Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia 2005; 7: 17–23.
Hruban RH, Maitra A, Schulick R, Laheru D, Herman J, Kern SE et al. Emerging molecular biology of pancreatic cancer. Gastrointest Cancer Res 2008; 2: S10–S15.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA . Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 2006; 20: 1218–1249.
Ben-Neriah Y, Karin M . Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol 2011; 12: 715–723.
Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ . The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res 1999; 5: 119–127.
Oeckinghaus A, Ghosh S . The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009; 1: a000034.
Baumgart S, Ellenrieder V, Fernandez-Zapico ME . Oncogenic transcription factors: cornerstones of inflammation-linked pancreatic carcinogenesis. Gut (e-pub ahead of print 13 October 2011, doi:10.1136/gutjnl-2011-301008).
Liptay S, Weber CK, Ludwig L, Wagner M, Adler G, Schmid RM . Mitogenic and antiapoptotic role of constitutive NF-kappaB/Rel activity in pancreatic cancer. Int J Cancer 2003; 105: 735–746.
Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T . NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett 2010; 295: 214–228.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003; 4: 437–450.
Herrera PL . Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development 2000; 127: 2317–2322.
Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Israel A, Rajewsky K et al. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell 2000; 5: 981–992.
Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 2001; 25: 579–586.
Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011; 19: 441–455.
Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011; 19: 456–469.
Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res 2011; 71: 5020–5029.
Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007; 11: 291–302.
Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernandez-Porras I, Canamero M et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 2011; 19: 728–739.
Funahashi H, Satake M, Dawson D, Huynh NA, Reber HA, Hines OJ et al. Delayed progression of pancreatic intraepithelial neoplasia in a conditional Kras(G12D) mouse model by a selective cyclooxygenase-2 inhibitor. Cancer Res 2007; 67: 7068–7071.
Fendrich V, Chen NM, Neef M, Waldmann J, Buchholz M, Feldmann G et al. The angiotensin-I-converting enzyme inhibitor enalapril and aspirin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Gut 2010; 59: 630–637.
Greer JB, Whitcomb DC . Inflammation and pancreatic cancer: an evidence-based review. Curr Opin Pharmacol 2009; 9: 411–418.
Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene 2006; 25: 2105–2112.
Aleksic T, Baumann B, Wagner M, Adler G, Wirth T, Weber CK . Cellular immune reaction in the pancreas is induced by constitutively active IkappaB kinase-2. Gut 2007; 56: 227–236.
Baumann B, Wagner M, Aleksic T, von Wichert G, Weber CK, Adler G et al. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J Clin Invest 2007; 117: 1502–1513.
Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H et al. Pten is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov 2011; 1: 158–169.
Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell 2012; 21: 105–120.
Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S et al. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest 2012; 122: 1519–1528.
Maniati E, Bossard M, Cook N, Candido JB, Emami-Shahri N, Nedospasov SA et al. Crosstalk between the canonical NF-kappaB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J Clin Invest 2011; 121: 4685–4699.
Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007; 446: 557–561.
Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R et al. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 2007; 11: 119–132.
Vlantis K, Wullaert A, Sasaki Y, Schmidt-Supprian M, Rajewsky K, Roskams T et al. Constitutive IKK2 activation in intestinal epithelial cells induces intestinal tumors in mice. J Clin Invest 2011; 121: 2781–2793.
Acknowledgements
We thank Melanie Gerstenlauer, Ute Leschik, Uta Manfras and Ursula Möhnle for technical assistance, and Pedro Herrera and Marc Schmidt-Supprian for providing mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Maier, H., Wagner, M., Schips, T. et al. Requirement of NEMO/IKKγ for effective expansion of KRAS-induced precancerous lesions in the pancreas. Oncogene 32, 2690–2695 (2013). https://doi.org/10.1038/onc.2012.272
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.272