Abstract
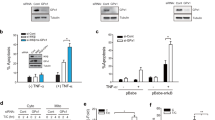
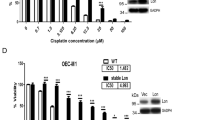
Mammalian Ste20-like kinase-1 (MST1) kinase mediates H2O2-induced cell death by anticancer drugs such as cisplatin in a p53-dependent manner. However, the mechanism underlying MST1 activation by H2O2 remains unknown. Here we show that peroxiredoxin-I (PRX-I) is an essential intermediate in H2O2-induced MST1 activation and cisplatin-induced cell death through p53. Cell stimulation with H2O2 resulted in PRX-I oxidation to form homo-oligomers and interaction with MST1, leading to MST1 autophosphorylation and augmentation of kinase activity. In addition, RNA interference knockdown experiments indicated that endogenous PRX-I is required for H2O2-induced MST1 activation. Live-cell imaging showed H2O2 generation by cisplatin treatment, which likewise caused PRX-I oligomer formation, MST1 activation and cell death. Cisplatin-induced PRX-I oligomer formation was not observed in embryonic fibroblasts obtained from p53-knockout mice, confirming the importance of p53. Indeed, ectopic expression of p53 induced PRX-I oligomer formation and cell death, both of which were cancelled by the antioxidant NAC. Moreover, we succeeded in reconstituting H2O2-induced MST1 activation in vitro, using purified PRX-I and MST1 proteins. Collectively, our results show a novel PRX-I function to cause cell death in response to high levels of oxidative stress by activating MST1, which underlies the p53-dependent cytotoxicity caused by anticancer agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ahn SH, Cheung WL, Hsu JY, Diaz RL, Smith MM, Allis CD . (2005). Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell 120: 25–36.
Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV et al. (2006). Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3: 281–286.
Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY et al. (2005). Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature 435: 347–353.
Colombani J, Polesello C, Josué F, Tapon N . (2006). Dmp53 activates the Hippo pathway to promote cell death in response to DNA damage. Curr Biol 16: 1453–1458.
Creasy CL, Ambrose DM, Chernoff J . (1996). The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem 271: 21049–21053.
de Souza PM, Lindsay MA . (2004). Mammalian Sterile20-like kinase 1 and the regulation of apoptosis. Biochem Soc Trans 32: 485–488.
Egler RA, Fernandes E, Rothermund K, Sereika S, de Souza-Pinto N, Jaruga P et al. (2005). Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene 24: 8038–8050.
Finkel T, Holbrook NJ . (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247.
Graves JA, Metukuri M, Scott D, Rothermund K, Prochownik EV . (2009). Regulation of reactive oxygen species homeostasis by peroxiredoxins and c-Myc. J Biol Chem 284: 6520–6529.
Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ . (1996). Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem 271: 4138–4142.
Harvey K, Tapon N . (2007). The Salvador–Warts–Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191.
Iwamura T, Yoneyama M, Yamaguchi K, Suhara W, Mori W, Shiota K et al. (2001). Induction of IRF-3/-7 kinase and NF-kappaB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6: 375–388.
Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH et al. (2004). Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117: 625–635.
Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T . (1996). Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA 93: 11848–11852.
Karin M, Hunter T . (1995). Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol 5: 747–757.
König J, Baier M, Horling F, Kahmann U, Harris G, Schürmann P et al. (2002). The plant-specific function of 2-Cys peroxiredoxin-mediated detoxification of peroxides in the redox-hierarchy of photosynthetic electron flux. Proc Natl Acad Sci USA 99: 5738–5743.
Lee W, Choi KS, Riddell J, Ip C, Ghosh D, Park JH et al. (2007). Human peroxiredoxin 1 and 2 are not duplicate proteins: the unique presence of CYS83 in Prx1 underscores the structural and functional differences between Prx1 and Prx2. J Biol Chem 282: 22011–22022.
Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villén J, Becker EB et al. (2006). A conserved MST–FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125: 987–1001.
Li PF, Dietz R, von Harsdorf R . (1999). p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J 18: 6027–6036.
Lowe SW, Bodis S, McClatchey A, Remington L, Ruley HE, Fisher DE et al. (1994). p53 status and the efficacy of cancer therapy in vivo. Science 266: 807–810.
Lowe SW, Ruley HE, Jacks T, Housman DE . (1993). p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967.
Moon JC, Hah YS, Kim WY, Jung BG, Jang HH, Lee JR et al. (2005). Oxidative stress-dependent structural and functional switching of a human 2-Cys peroxiredoxin isotype II that enhances HeLa cell resistance to H2O2-induced cell death. J Biol Chem 280: 28775–28784.
Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL et al. (2003). Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature 424: 561–565.
O'Neill EE, Matallanas D, Kolch W . (2005). Mammalian sterile 20-like kinases in tumor suppression: an emerging pathway. Cancer Res 65: 5485–5487.
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B . (1997). A model for p53-induced apoptosis. Nature 389: 300–305.
Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J . (2004). Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J 381: 453–462.
Praskova M, Xia F, Avruch J . (2008). MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 18: 311–321.
Ren A, Yan G, You B, Sun J . (2008). Downregulation of mammalian sterile 20-like kinase 1 by heat shock protein 70 mediates cisplatin resistance in prostate cancer cells. Cancer Res 68: 2266–2274.
Rhee SG, Chae HZ, Kim K . (2005). Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38: 1543–1552.
Rhee SG . (2006). Cell signaling. H2O2, a necessary evil for cell signaling. Science 312: 1882–1883.
Trachootham D, Alexandre J, Huang P . (2009). Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8: 579–591.
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H et al. (2006). Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 10: 241–252.
Tsukada T, Tomooka Y, Takai S, Ueda Y, Nishikawa S, Yagi T et al. (1993). Enhanced proliferative potential in culture of cells from p53-deficient mice. Oncogene 8: 3313–3322.
Veal EA, Day AM, Morgan BA . (2007). Hydrogen peroxide sensing and signaling. Mol Cell 26: 1–14.
Veal EA, Findlay VJ, Day AM, Bozonet SM, Evans JM, Quinn J et al. (2004). A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell 15: 129–139.
Wood ZA, Schröder E, Robin Harris J, Poole LB . (2003). Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci 28: 32–40.
Zeng Q, Hong W . (2008). The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell 13: 188–192.
Acknowledgements
We thank Dr Yoshiaki Kise (Flanders Institute for Biotechnology, Belgium) for technical assistance in preparing the expression constructs. This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS) and Ministry of Education, Culture, Sports, Science and Technology (MEXT)-Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Morinaka, A., Funato, Y., Uesugi, K. et al. Oligomeric peroxiredoxin-I is an essential intermediate for p53 to activate MST1 kinase and apoptosis. Oncogene 30, 4208–4218 (2011). https://doi.org/10.1038/onc.2011.139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.139
Keywords
This article is cited by
-
A key metabolic integrator, coenzyme A, modulates the activity of peroxiredoxin 5 via covalent modification
Molecular and Cellular Biochemistry (2019)
-
PRX1 knockdown potentiates vitamin K3 toxicity in cancer cells: a potential new therapeutic perspective for an old drug
Journal of Experimental & Clinical Cancer Research (2015)
-
Mst1 and Mst2 kinases: regulations and diseases
Cell & Bioscience (2013)
-
Germinal center kinases in immune regulation
Cellular & Molecular Immunology (2012)