Abstract
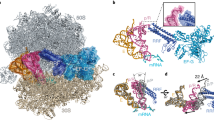
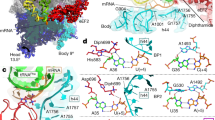
EF4 catalyzes tRNA back-translocation through an unknown mechanism. We report cryo-EM structures of Escherichia coli EF4 in post- and pretranslocational ribosomes (Post– and Pre–EF4) at 3.7- and 3.2-Å resolution, respectively. In Post–EF4, peptidyl-tRNA occupies the peptidyl (P) site, but the interaction between its CCA end and the P loop is disrupted. In Pre–EF4, the peptidyl-tRNA assumes a unique position near the aminoacyl (A) site, denoted the A site/EF4 bound (A/4) site, with a large displacement at its acceptor arm. Mutagenesis analyses suggest that a specific region in the EF4 C-terminal domain (CTD) interferes with base-pairing between the peptidyl-tRNA 3′-CCA and the P loop, whereas the EF4 CTD enhances peptidyl-tRNA interaction at the A/4 site. Therefore, EF4 induces back-translocation by disengaging the tRNA's CCA end from the peptidyl transferase center of the translating ribosome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Liu, G. et al. EF-G catalyzes tRNA translocation by disrupting interactions between decoding center and codon-anticodon duplex. Nat. Struct. Mol. Biol. 21, 817–824 (2014).
Qin, Y. et al. The highly conserved LepA is a ribosomal elongation factor that back-translocates the ribosome. Cell 127, 721–733 (2006).
Liu, H., Pan, D., Pech, M. & Cooperman, B.S. Interrupted catalysis: the EF4 (LepA) effect on back-translocation. J. Mol. Biol. 396, 1043–1052 (2010).
Liu, H. et al. The conserved protein EF4 (LepA) modulates the elongation cycle of protein synthesis. Proc. Natl. Acad. Sci. USA 108, 16223–16228 (2011).
Evans, R.N., Blaha, G., Bailey, S. & Steitz, T.A. The structure of LepA, the ribosomal back translocase. Proc. Natl. Acad. Sci. USA 105, 4673–4678 (2008).
Yamamoto, H. et al. EF-G and EF4: translocation and back-translocation on the bacterial ribosome. Nat. Rev. Microbiol. 12, 89–100 (2014).
Han, B. & Qin, Y. Bioinformatics analysis reveals that LepA C-terminal domain is highly conserved in domain architectures and phylogenetic distribution. Scientia Sinica Chimica 42, 24–31 (2012).
Zhang, D. & Qin, Y. The paradox of elongation factor 4: highly conserved, yet of no physiological significance? Biochem. J. 452, 173–181 (2013).
Connell, S.R. et al. A new tRNA intermediate revealed on the ribosome during EF4-mediated back-translocation. Nat. Struct. Mol. Biol. 15, 910–915 (2008).
Gagnon, M.G., Lin, J., Bulkley, D. & Steitz, T.A. Crystal structure of elongation factor 4 bound to a clockwise ratcheted ribosome. Science 345, 684–687 (2014).
Selmer, M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006).
Pulk, A. & Cate, J.H. Control of ribosomal subunit rotation by elongation factor G. Science 340, 1235970 (2013).
De Laurentiis, E.I. & Wieden, H.J. Identification of two structural elements important for ribosome-dependent GTPase activity of elongation factor 4 (EF4/LepA). Sci. Rep. 5, 8573 (2015).
Spirin, A.S. The ribosome as a conveying thermal ratchet machine. J. Biol. Chem. 284, 21103–21119 (2009).
Moore, P.B. How should we think about the ribosome? Annu. Rev. Biophys. 41, 1–19 (2012).
Konevega, A.L. et al. Spontaneous reverse movement of mRNA-bound tRNA through the ribosome. Nat. Struct. Mol. Biol. 14, 318–324 (2007).
Shoji, S., Walker, S.E. & Fredrick, K. Reverse translocation of tRNA in the ribosome. Mol. Cell 24, 931–942 (2006).
Frank, J. & Gonzalez, R.L. Jr. Structure and dynamics of a processive Brownian motor: the translating ribosome. Annu. Rev. Biochem. 79, 381–412 (2010).
Dashti, A. et al. Trajectories of the ribosome as a Brownian nanomachine. Proc. Natl. Acad. Sci. USA 111, 17492–17497 (2014).
Valle, M. et al. Locking and unlocking of ribosomal motions. Cell 114, 123–134 (2003).
Frank, J., Gao, H., Sengupta, J., Gao, N. & Taylor, D.J. The process of mRNA-tRNA translocation. Proc. Natl. Acad. Sci. USA 104, 19671–19678 (2007).
Zhou, J., Lancaster, L., Donohue, J.P. & Noller, H.F. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 345, 1188–1191 (2014).
Balakrishnan, R., Oman, K., Shoji, S., Bundschuh, R. & Fredrick, K. The conserved GTPase LepA contributes mainly to translation initiation in Escherichia coli. Nucleic Acids Res. 42, 13370–13383 (2014).
Yang, F., Li, Z., Hao, J. & Qin, Y. EF4 knockout E. coli cells exhibit lower levels of cellular biosynthesis under acidic stress. Protein Cell 5, 563–567 (2014).
Pech, M. et al. Elongation factor 4 (EF4/LepA) accelerates protein synthesis at increased Mg2+ concentrations. Proc. Natl. Acad. Sci. USA 108, 3199–3203 (2011).
Wang, L. et al. A conserved proline switch on the ribosome facilitates the recruitment and binding of trGTPases. Nat. Struct. Mol. Biol. 19, 403–410 (2012).
Zhang, D. et al. Common chaperone activity in the G-domain of trGTPase protects L11-L12 interaction on the ribosome. Nucleic Acids Res. 40, 10851–10865 (2012).
Dibb, N.J. & Wolfe, P.B. lep operon proximal gene is not required for growth or secretion by Escherichia coli. J. Bacteriol. 166, 83–87 (1986).
Bijlsma, J.J., Lie-A-Ling, M., Nootenboom, I.C., Vandenbroucke-Grauls, C.M. & Kusters, J.G. Identification of loci essential for the growth of Helicobacter pylori under acidic conditions. J. Infect. Dis. 182, 1566–1569 (2000).
Jenner, L.B., Demeshkina, N., Yusupova, G. & Yusupov, M. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat. Struct. Mol. Biol. 17, 555–560 (2010).
Mikkelsen, N.E., Johansson, K., Virtanen, A. & Kirsebom, L.A. Aminoglycoside binding displaces a divalent metal ion in a tRNA-neomycin B complex. Nat. Struct. Biol. 8, 510–514 (2001).
Schmeing, T.M. et al. The crystal structure of the ribosome bound to EF-Tu and aminoacyl-tRNA. Science 326, 688–694 (2009).
Walker, S.E., Shoji, S., Pan, D., Cooperman, B.S. & Fredrick, K. Role of hybrid tRNA-binding states in ribosomal translocation. Proc. Natl. Acad. Sci. USA 105, 9192–9197 (2008).
Márquez, V., Wilson, D.N., Tate, W.P., Triana-Alonso, F. & Nierhaus, K.H. Maintaining the ribosomal reading frame: the influence of the E site during translational regulation of release factor 2. Cell 118, 45–55 (2004).
Shaikh, T.R. et al. SPIDER image processing for single-particle reconstruction of biological macromolecules from electron micrographs. Nat. Protoc. 3, 1941–1974 (2008).
Mindell, J.A. & Grigorieff, N. Accurate determination of local defocus and specimen tilt in electron microscopy. J. Struct. Biol. 142, 334–347 (2003).
Scheres, S.H. A Bayesian view on cryo-EM structure determination. J. Mol. Biol. 415, 406–418 (2012).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Rosenthal, P.B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Goddard, T.D., Huang, C.C. & Ferrin, T.E. Visualizing density maps with UCSF Chimera. J. Struct. Biol. 157, 281–287 (2007).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Chen, V.B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
Connell, S.R. et al. Mechanism of Tet(O)-mediated tetracycline resistance. EMBO J. 22, 945–953 (2003).
Phillips, J.C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Hornak, V. et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65, 712–725 (2006).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14 33–38, 27–28 (1996).
Meagher, K.L., Redman, L.T. & Carlson, H.A. Development of polyphosphate parameters for use with the AMBER force field. J. Comput. Chem. 24, 1016–1025 (2003).
Essmann, U. et al. A Smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an Nċlog(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Gutierrez, E. et al. eIF5A promotes translation of polyproline motifs. Mol. Cell 51, 35–45 (2013).
Lancaster, L., Kiel, M.C., Kaji, A. & Noller, H.F. Orientation of ribosome recycling factor in the ribosome from directed hydroxyl radical probing. Cell 111, 129–140 (2002).
Li, W. & Godzik, A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22, 1658–1659 (2006).
Edgar, R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Crooks, G.E., Hon, G., Chandonia, J.M. & Brenner, S.E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Plotree, D. & Plotgram, D. PHYLIP-phylogeny inference package (version 3.2). Cladistics 5, 163–166 (1989).
Acknowledgements
We thank X. Fu for discussion and English editing, and the members of P. Zhu's laboratory for help and discussions. Y.Q. is supported by an Institute of Biophysics 135 Goal-oriented Project and the Key Laboratory of RNA Biology (Institute of Biophysics, Chinese Academy of Sciences). This work was supported by grants from the Ministry of Science and Technology of China (2012CB911000 and 2013CB531200 to Y.Q.; 2013CB910404 to N.G.), the National Natural Science Foundation of China (31322015, 31170756 and 31270847 to Y.Q.; 31422016 and 31470722 to N.G.) and the Strategic Priority Research program of the Chinese Academy of Sciences (XDB08010203 to Y.Q.).
Author information
Authors and Affiliations
Contributions
D.Z., G.L. and G.S. cloned constructs, performed biochemical assays and prepared cryo-EM specimens. K.Y., E.C. and S.W. collected cryo-EM data. K.Y. and N.G. analyzed the cryo-EM data and reconstituted the structures. Y.S. and J.L. performed MD simulations. J.L. and T.J. performed bioinformatics analyses. Y.Q. and N.G. analyzed all data and wrote the manuscript. All authors discussed the results and commented on the manuscript. Y.Q. directed and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Comparisons of EF4 with EF-G.
(a) Comparison of the domain structures of EF4 and EF-G. (b) Comparison of the interaction of EF-G with tRNAs in the Post complex (PDB 4V5F, Gao, Y.G. et al. Science 326, 694-9 (2009).) and that of EF4 with tRNAs in the Pre complex (PDB 3DEG, Connell, S.R. et al. Nat Struct Mol Biol 15, 910-5 (2008).). The mutual domains of EF-G and EF4, namely domains I, II, III and V, compose the ‘common domain’ (grey area) that only contacts the ribosome. The factor-specific domain, namely domain IV of EF-G or EF4-CTD, is the ‘direction domain’ (blue area) that contacts peptidyl-tRNA (and mRNA) and is responsible for forward (green arrow) or backward (red arrow) translocation, respectively. The molecular mechanism of EF-G-catalyzed forward translocation has been clarified recently (Liu, G. et al. Nat Struct Mol Biol 21, 817-24 (2014).). Yet, the EF4 working mechanism remains elusive. (c) An unrooted phylogenetic tree of the EF4 and EF-G was constructed using the neighbor-joining method with Phylip program. Red branches are EF4 and blue branches are EF-G. Branch lengths reflect the estimated amino acid substitutions per every site (scale bar in the middle). In general, EF4 and EF-G belong to two clades.
Supplementary Figure 2 Sample preparation and classification of the translating ribosomal complexes with EF4.
(a) Binding of EF4 to the Post complex programmed by sequence specific mRNA and cognate tRNAs in the P and E sites. The sample was prepared in the presence of GDPNP. Pre: before sucrose cushion. Su: supernatant after sucrose cushion. (b) Puromycin activity of the cryo-EM specimen, namely the Post complex in the presence of EF4 with GTP or GDPNP. The puromycin activity of Pre complex in the presence of EF-G−GTP was considered as 100 %. Error bars, s.e.m., n=5. (c) RELION-based 3D classification of Post−EF4−GDPNP particles. Pre−EF4 are indicated in red box, Post−EF4 in blue box. Unit, 1,000. (d−g) Cryo-EM density of EF4-bound ribosomal complexes and the resolutions of the reconstituted structures. (d,e) Surface view of the final maps of Post−EF4 complex (d) and Pre−EF4 complex (e). (f,g) Fourier Shell Correlation (FSC) curve for the final structures. The resolution of Post−EF4 complex (f) and Pre−EF4 complex (g) is 3.74 Å and 3.22 Å, respectively, FSC=0.143. (h,i) Density maps of the CCA-end of the P-tRNA (yellow) and the P-loop (blue) in Post–EF4 (h) and Pre–EF4 (i) complexes, at 3σ and 4σ levels.
Supplementary Figure 3 Interactions between EF4 and the shoulder of 30S in Pre−EF4, and structural comparison of EF4 extracted from Pre−EF4 and previous structures.
(a−c) In Pre−EF4, the nethermost domain of EF4 tending towards small subunit is domain II. The most distal tip of this domain has a tyrosine residue (Tyr203) stacks with A55 of 16S, which is in the shoulder region of small subunit. However, in the crystal structure of T. thermophiles 70S–EF4 complexes (PDB 4W2E, Gagnon, M.G., Lin, J., Bulkley, D. & Steitz, T.A. Science 345, 684-7 (2014).), A55 and Y203 was indicated far from each other. (d–f) EF4 extracted from Pre−EF4 (multicolored) is aligned to the G-domain of E. coli EF4 crystal structure (PDB 3CB4, Evans, R.N., Blaha, G., Bailey, S. & Steitz, T.A. Proc Natl Acad Sci U S A 105, 4673-8 (2008).) (gray) (d), the EF4 (green) of E. coli 70S−EF4 (PDB 3DEG, Connell, S.R. et al. Nat Struct Mol Biol 15, 910-5 (2008).) (e), or the EF4 (light blue) of T. thermophiles 70S−EF4 (PDB 4QJT, Gagnon, M.G., Lin, J., Bulkley, D. & Steitz, T.A. Science 345, 684-7 (2014).) (f). The individual domain of EF4 from Pre−EF4 is colored as follows. Light gray: G-domain and domain II; blue: domain III; yellow: domain IV; magenta: domain IV extension; orange: CTD.
Supplementary Figure 4 Structural comparison of the GTPase motif in EF4 and EF-G.
(a) Surface representation of the cryo-EM density map of the EF4 in Pre−EF4 with atomic model superimposed. White: G-domain; gray: domain II; blue: domain III; yellow: domain IV; magenta: domain IV extension; orange: CTD. In G-domain, green: switch I (SW I); red: switch II (SW II). (b) Structural comparison of the G-domains. Blue: Pre−EF4 of this study; white: EF-G−GMPCP in the E. coli ribosome−EF-G structure (PDB 4V9O, Pulk, A. & Cate, J.H. Science 340, 1235970 (2013).); light blue: EF4−GDP in the T. thermophiles ribosome−EF4 structure (PDB 4QJT, Gagnon, M.G., Lin, J., Bulkley, D. & Steitz, T.A. Science 345, 684-7 (2014).). The switches are colored as in (a). (c,d) Structural arrangement of domain II (gray), III (blue) and IV (yellow), when EF4 (in Pre−EF4 of this study) and EF4−GDP (PDB 4QJT, Gagnon, M.G., Lin, J., Bulkley, D. & Steitz, T.A. Science 345, 684-7 (2014).) (c) or EF-G−GMPCP (PDB 4V9O, Pulk, A. & Cate, J.H. Science 340, 1235970 (2013).) were aligned to their G-domain (d). (e,f) Structural comparison as in (c,d), but aligned to both the G-domain and domain II.
Supplementary Figure 5 Effects of EF4 CTD mutations on factor binding, GTPase activity and back-translocation of tRNA.
(a) Chemical footprinting of ribosome−EF4−GDPNP complexes to detect the interactions between EF4 and SRL (upper) or GAC (lower). Because the G domain and domain IV of EF4 are in contact with the SRL and the GAC of 23S rRNA, respectively, we tested the ability of EF4 mutants to protect both A2660 (SRL) and A1067 (GAC). The tRNA interacting sites are indicated above the mutants. A and C: dideoxy-sequencing lanes. Relative quantification of the bands corresponding to A2660 or A1067 is shown below the gel. (b) Multiple turnover of GTP hydrolysis in the presence of EF4 or its mutants. The tRNA interacting sites are indicated beneath the mutants. Error bars, s.e.m., n = 5. ***P < 0.001 by two-tailed Student's t test. (c−e) EF4-CTD mutants decrease the back-translocation of tRNA. Chemical footprinting of ribosome−EF4−GDPNP complexes to detect the interactions between tRNAs and A site (c), P site (d), or E site (e) in the presence of EF4 or its mutants. A and C: dideoxy-sequencing lanes. Quantification of the probing signals is shown below the gel. The empty 70S ribosome in the presence of DMS was taken as references (1.0). Error bars, s.e.m., n = 5, ***P < 0.001 by two-tailed Student's t test.
Supplementary Figure 6 Interactions of EF4 CTD with the PTC.
(a) Weblogo representation of the multiple sequence alignment of the regions of EF4 interacting with the PTC. On the Y-axis scores are shown in bits, the X-axis shows the position in the alignment. Bits are calculated in log2-scale. Since there are 20 amino acids, the maximum bits-value is log220 = 4.32. In a sequence logo, the height of amino acids reflects its frequency in the alignment and is presented in bits. The residues of functional essentiality are highlighted in yellow. (b) Positions of single cysteine mutations (blue) in EF4 protein and its interaction with the elements of the PTC. A-loop: cyan; P-loop: blue; A2602: magenta. (c) Directed hydroxyl radical probing of the PTC of the Fe(II)-Post−EF4 complexes by primer extension experiments. T. thermophilus EF4 has been used here and the residue number in corresponding E. coli homologue is indicated in the brackets. Red colored residues showed significant probing variation upon EF4 binding. A, U, G and C indicate sequencing lanes. (d) Quantification of G2253 and A2602 bands in (c). The band intensity without Fe(II)-EF4 (no C) was set as reference (1.0). Data are represented as mean ± s.e.m., n = 5, **P < 0.01 by two-tailed Student's t test. (e) Relative back-TL activity of EF4 R560 tip mutants. The relative back-TL is given as puromycin reaction of the Post complex in the presence EF4 wt (100%) or its mutants. Error bars, s.e.m., n = 5, ***P < 0.001 by two-tailed Student's t test.
Supplementary Figure 7 Phylogenetic analysis of EF4 CTD and EF-G domain IV.
Weblogo representation of the multiple sequence alignment of EF4-CTD (a) and EF-G domain IV (b). On the Y-axis scores are shown in bits, the X-axis shows the position in the alignment. Bits are calculated in log2-scale. Since there are 20 amino acids, the maximum bits-value is log220 = 4.32. In a sequence logo, the height of amino acids reflects its frequency in the alignment and is presented in bits. In EF4 CTD, most residues are positively charged and highly conserved; in contrast, EF-G domain IV has much less conserved residues and they are normally uncharged.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1827 kb)
Supplementary Data Set 1
Uncropped image of the urea-PAGE gel shown in Fig. 4g (PDF 136 kb)
Interaction of EF4 with A/4-tRNA
Density map of A/4-tRNA and EF4 in PRE–EF4. Gold: EF4; red: EF4-CTD; green: A/4- tRNA (MOV 20092 kb)
Rights and permissions
About this article
Cite this article
Zhang, D., Yan, K., Liu, G. et al. EF4 disengages the peptidyl-tRNA CCA end and facilitates back-translocation on the 70S ribosome. Nat Struct Mol Biol 23, 125–131 (2016). https://doi.org/10.1038/nsmb.3160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.3160
This article is cited by
-
Mammalian elongation factor 4 regulates mitochondrial translation essential for spermatogenesis
Nature Structural & Molecular Biology (2016)
-
Step back for seminal translation
Nature Structural & Molecular Biology (2016)