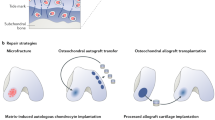
Key Points
-
Scaffolds are an essential component of the tissue engineering triad concept and should provide form, fixation and function, as well as drive tissue formation
-
Natural scaffold materials are highly biocompatible, biodegradable and have multiple attachment sites for cells
-
Synthetic scaffolds can be manufactured to have highly predictive properties
-
Scaffolds for the regeneration of articular cartilage, tendon and meniscus have been investigated in many clinical trials, but there is no consensus on the best material or technique
-
Clinical study of scaffold-based bone and skeletal muscle tissue engineering has been limited by the need for vascularization and the complex physiological and biomechanical requirements of these tissues
-
The other two elements of the tissue engineering triad, cells and growth factors, have a role in the clinical application of scaffold-based musculoskeletal tissue engineering
Abstract
Musculoskeletal disease and injury are highly prevalent conditions that lead to many surgical procedures. Autologous tissue transfer, allograft transplantation and nontissue prosthetics are currently used for the surgical treatment of critical-sized defects. However, the field of tissue engineering is actively investigating tissue-replacement solutions, many of which involve 3D scaffolds. Scaffolds must provide a balance of shape, biomechanical function and biocompatibility in order to achieve tissue replacement success. Different tissues can have different requirements for success, which has led to the development of various materials with unique characteristics. Articular cartilage scaffolds have the most robust clinical experience, with many scaffolds, mostly constructed of natural materials, showing promise, but levels of success vary. Tendon scaffolds also have proven clinical applications, with human-dermis-derived scaffolds showing the most potential. Synthetic and naturally derived meniscus scaffolds have been investigated in few clinical studies, but the results are encouraging. Bone scaffolds are limited to amorphous pastes and putties, owing to difficulties achieving adequate vascularization and biomechanical optimization. The complex physiological function and vascular demands of skeletal muscle have limited the widespread clinical use of scaffolds for engineering this tissue. Continued progress in preclinical study, not only of scaffolds, but also of other facets of tissue engineering, should enable the successful translation of musculoskeletal tissue engineering solutions to the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States, 2nd edn (American Academy of Orthopaedic Surgeons, Rosemont, 2011).
Gamie, Z. et al. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin. Biol. Ther. 12, 713–729 (2012).
Li, M. T., Willett, N. J., Uhrig, B. A., Guldberg, R. E. & Warren, G. L. Functional analysis of limb recovery following autograft treatment of volumetric muscle loss in the quadriceps femoris. J. Biomech. 47, 2013–2021 (2014).
Mariscalco, M. W. et al. Autograft versus nonirradiated allograft tissue for anterior cruciate ligament reconstruction: a systematic review. Am. J. Sports Med. 42, 492–499 (2014).
Shrivats, A. R., Mcdermott, M. C. & Hollinger, J. O. Bone tissue engineering: state of the union. Drug Discov. Today 19, 781–786 (2014).
Zmistowski, B., Karam, J. A., Durinka, J. B., Casper, D. S. & Parvizi, J. Periprosthetic joint infection increases the risk of one-year mortality. J. Bone Joint Surg. Am. 95, 2177–2184 (2013).
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Murphy, C. M., O'Brien, F. J., Little, D. G. & Schindeler, A. Cell–scaffold interactions in the bone tissue engineering triad. Eur. Cell. Mater. 26, 120–132 (2013).
Hollister, S. J. & Murphy, W. L. Scaffold translation: barriers between concept and clinic. Tissue Eng. Part B Rev. 17, 459–474 (2011).
Scotti, C., Hirschmann, M. T., Antinolfi, P., Martin, I. & Peretti, G. M. Meniscus repair and regeneration: review on current methods and research potential. Eur. Cell. Mater. 26, 150–170 (2013).
Behrens, P., Bitter, T., Kurz, B. & Russlies, M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee 13, 194–202 (2006).
Ronga, M., Grassi, F. A., Manelli, A. & Bulgheroni, P. Tissue engineering techniques for the treatment of a complex knee injury. Arthroscopy 22, 576.e1–576.e3 (2006).
Gigante, A. et al. Distal realignment and patellar autologous chondrocyte implantation: mid-term results in a selected population. Knee Surg. Sports Traumatol. Arthrosc. 17, 2–10 (2009).
Basad, E., Ishaque, B., Bachmann, G., Stürz, H. & Steinmeyer, J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg. Sports Traumatol. Arthrosc. 18, 519–527 (2010).
Macmull, S. et al. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int. Orthop. 36, 1371–1377 (2012).
Bauer, S. et al. Knee joint preservation with combined neutralising high tibial osteotomy (HTO) and matrix-induced autologous chondrocyte implantation (MACI) in younger patients with medial knee osteoarthritis: a case series with prospective clinical and MRI follow-up over 5 years. Knee 19, 431–439 (2012).
Ventura, A. et al. Repair of osteochondral lesions in the knee by chondrocyte implantation using the MACI® technique. Knee Surg. Sports Traumatol. Arthrosc. 20, 121–126 (2012).
Gille, J. et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch. Orthop. Trauma Surg. 133, 87–93 (2013).
Pascarella, A. et al. Treatment of articular cartilage lesions of the knee joint using a modified AMIC technique. Knee Surg. Sports Traumatol. Arthrosc. 18, 509–513 (2010).
Dhollander, A. A. et al. Autologous matrix-induced chondrogenesis combined with platelet-rich plasma gel: technical description and a five pilot patients report. Knee Surg. Sports Traumatol. Arthrosc. 19, 536–542 (2011).
Schiavone Panni, A., Cerciello, S. & Vasso, M. The manangement of knee cartilage defects with modified amic technique: preliminary results. Int. J. Immunopathol. Pharmacol. 24 (1 Suppl. 2), 149–152 (2011).
Kusano, T. et al. Treatment of isolated chondral and osteochondral defects in the knee by autologous matrix-induced chondrogenesis (AMIC). Knee Surg. Sports Traumatol. Arthrosc. 20, 2109–2115 (2012).
Nehrer, S. et al. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur. J. Radiol. 57, 3–8 (2006).
Ferruzzi, A. et al. Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J. Bone Joint Surg. Am. 90 (Suppl. 4), 90–101 (2008).
Kon, E. et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am. J. Sports Med. 37, 33–41 (2009).
Gobbi, A. et al. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years' follow-up. Am. J. Sports Med. 37, 1083–1092 (2009).
Clar, H. et al. Matrix-assisted autologous chondrocyte implantation into a 14cm2 cartilage defect, caused by steroid-induced osteonecrosis. Knee 17, 255–257 (2010).
Kon, E. et al. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur. J. Radiol. 79, 382–388 (2011).
Kon, E. et al. Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am. J. Sports Med. 39, 2549–2557 (2011).
Kon, E. V. et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am. J. Sports Med. 39, 1668–1675 (2011).
Filardo G. et al. Arthroscopic second generation autologous chondrocytes implantation associated with bone grafting for the treatment of knee osteochondritis dissecans: results at 6 years. Knee 19, 658–663 (2012).
Brix, M. O. et al. Treatment of full-thickness chondral defects with hyalograft C in the knee: long-term results. Am. J. Sports Med. 42, 1426–1432 (2014).
Nehrer, S., Dorotka, R., Domayer, S., Stelzeneder, D. & Kotz, R. Treatment of full-thickness chondral defects with hyalograft C in the knee: a prospective clinical case series with 2 to 7 years' follow-up. Am. J. Sports Med. 37 (Suppl. 1), 81S–87S (2009).
Filardo, G., Kon, E., Di Martino, A., Iacono, F. & Marcacci, M. Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am. J. Sports Med. 39, 2153–2160 (2011).
Filardo, G. et al. Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg. Sports Traumatol. Arthrosc. 20, 1704–1713 (2012).
De Windt, T. S., Concaro, S., Lindahl, A., Saris, D. B. & Brittberg, M. Strategies for patient profiling in articular cartilage repair of the knee: a prospective cohort of patients treated by one experienced cartilage surgeon. Knee Surg. Sports Traumatol. Arthrosc. 20, 2225–2232 (2012).
Chevrier, A., Hoemann, C. D., Sun, J. & Buschmann, M. D. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage 15, 316–327 (2007).
Stanish, W. D. et al. Novel scaffold-based BST-CarGel treatment results in superior cartilage repair compared with microfracture in a randomized controlled trial. J. Bone Joint Surg. Am. 95, 1640–1650 (2013).
Steinwachs, M. R., Waibl, B. & Mumme, M. Arthroscopic treatment of cartilage lesions with microfracture and BST-CarGel. Arthrosc. Tech. 3, e399–e402 (2014).
Ossendorf, C. et al. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res. Ther. 9, R41 (2007).
Kreuz, P. C., Müller, S., Ossendorf, C., Kaps, C. & Erggelet, C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res. Ther. 11, R33 (2009).
Erggelet, C. et al. Autologous chondrocyte implantation versus ACI using 3D-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch. Orthop. Trauma Surg. 130, 957–964 (2010).
Kreuz, P. C. et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am. J. Sports Med. 39, 1697–1705 (2011).
Zeifang, F. et al. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am. J. Sports Med. 38, 924–933 (2010).
Nukavarapu, S. P. & Dorcemus, D. L. Osteochondral tissue engineering: current strategies and challenges. Biotechnol. Adv. 31, 706–721 (2013).
US National Institutes of Health. ClinicalTrials.gov [online], (2014).
Filardo, G. et al. Osteochondral scaffold reconstruction for complex knee lesions: a comparative evaluation. Knee 20, 570–576 (2013).
Steele, J. A. et al. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 10, 2065–2075 (2014).
Joshi, N., Reverte-vinaixa, M., Díaz-Ferreiro, E. W. & Domínguez-Oronoz, R. Synthetic resorbable scaffolds for the treatment of isolated patellofemoral cartilage defects in young patients: magnetic resonance imaging and clinical evaluation. Am. J. Sports Med. 40, 1289–1295 (2012).
Gott, M. et al. Tendon phenotype should dictate tissue engineering modality in tendon repair: a review. Discov. Med. 12, 75–84 (2011).
Ricchetti, E. T., Aurora, A., Iannotti, J. P. & Derwin, K. A. Scaffold devices for rotator cuff repair. J. Shoulder Elbow Surg. 21, 251–265 (2012).
Barber, F. A., Burns, J. P., Deutsch, A., Labbé, M. R. & Litchfield, R. B. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28, 8–15 (2012).
Dopirak, R., Bond, J. L. & Snyder, S. J. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Int. J. Shoulder Surg. 1, 7–15 (2007).
Burkhead, W. Z., Schiffern, S. C. & Krishnan, S. G. Use of GraftJacket as an augmentation for massive rotator cuff tears. Semin. Arthroplasty 18, 11–18 (2007).
Bond, J. L., Dopirak, R. M., Higgins, J., Burns, J. & Snyder, S. J. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy 24, 403.e1–403.e8 (2008).
Wong, I., Burns, J. & Snyder, S. Arthroscopic GraftJacket repair of rotator cuff tears. J. Shoulder Elbow Surg. 19 (2 Suppl.), 104–109 (2010).
Metcalf, M. H., Savoie, F. H. III & Kellum, B. Surgical technique for xeno-graft (SIS) augmentation of rotator-cuff repairs. Oper. Tech. Orthop. 12, 204–208 (2002).
Sclamberg, S. G., Tibone, J. E., Itamura, J. M. & Kasraeian, S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. J. Shoulder Elbow Surg. 13, 538–541 (2004).
Iannotti, J. P. et al. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J. Bone Joint Surg. Am. 88, 1238–1244 (2006).
Malcarney, H. L., Bonar, F. & Murrell, G. A. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am. J. Sports Med. 33, 907–911 (2005).
Walton, J. R., Bowman, N. K., Khatib, Y., Linklater, J. & Murrell, G. A. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J. Bone Joint Surg. Am. 89, 786–791 (2007).
Daly, K. A. et al. Effect of the αGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng. Part A 15, 3877–3888 (2009).
Xu, H. et al. A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-α-(1,3)-galactose and retention of matrix structure. Tissue Eng. Part A 15, 1807–1819 (2009).
Proctor, C. S. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. J. Shoulder Elbow Surg. 23, 1508–1513 (2014).
Encalada-Diaz, I. et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months' follow-up. J. Shoulder Elbow Surg. 20, 788–794 (2011).
Zhang, X., Bogdanowicz, D., Erisken, C., Lee, N. M. & Lu, H. H. Biomimetic scaffold design for functional and integrative tendon repair. J. Shoulder Elbow Surg. 21, 266–277 (2012).
Lu, H. H. & Thomopoulos, S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 15, 201–226 (2013).
Moffat, K. L. et al. In vitro and in vivo evaluation of a bi-phasic nanofiber scaffold for integrative rotator cuff repair [poster #482]. Transactions of Orthopeadic Research Society 2011 Annual Meeting [online], (2011).
Nguyen, L. H. et al. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng. Part B Rev. 18, 363–382 (2012).
Oryan, A., Alidadi, S., Moshiri, A. & Maffulli, N. Bone regenerative medicine: classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 9, 18 (2014).
Smucker, J. D., Petersen, E. B. & Fredericks, D. C. Assessment of Mastergraft putty as a graft extender in a rabbit posterolateral fusion model. Spine (Phila. PA 1976) 37, 1017–1021 (2012).
Miller, C. P. et al. The efficacies of 2 ceramic bone graft extenders for promoting spinal fusion in a rabbit bone paucity model. Spine (Phila. PA 1976) 37, 642–647 (2012).
Kapur, R. A. et al. Clinical outcomes and fusion success associated with the use of BoneSave in spinal surgery. Arch. Orthop. Trauma Surg. 130, 641–647 (2010).
Blom, A. W. et al. Impaction bone grafting of the acetabulum at hip revision using a mix of bone chips and a biphasic porous ceramic bone graft substitute. Acta Orthop. 80, 150–154 (2009).
Whitehouse, M. R., Dacombe, P. J., Webb, J. C. & Blom, A. W. Impaction grafting of the acetabulum with ceramic bone graft substitute mixed with femoral head allograft: high survivorship in 43 patients with a median follow-up of 7 years: a follow-up report. Acta Orthop. 84, 365–370 (2013).
Burkus, J. K., Gornet, M. F., Dickman, C. A. & Zdeblick, T. A. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J. Spinal Disord. Tech. 15, 337–349 (2002).
Burkus, J. K., Transfeldt, E. E., Kitchel, S. H., Watkins, R. G. & Balderston, R. A. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila. PA 1976) 27, 2396–2408 (2002).
Govender, S. et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am. 84, 2123–2134 (2002).
Hashmi, S., Noureldin, M. & Khan, S. N. Lessons from the infuse trials: do we need a classification of bias in scientific publications and editorials? Curr. Rev. Musculoskelet. Med. 7, 193–199 (2014).
Moreau, J. L., Weir, M. D. & Xu, H. H. Self-setting collagen-calcium phosphate bone cement: mechanical and cellular properties. J. Biomed. Mater. Res. A 91, 605–613 (2009).
van staden, A. D. & Dicks, L. M. Calcium orthophosphate-based bone cements (CPCs): applications, antibiotic release and alternatives to antibiotics. J. Appl. Biomater. Funct. Mater. 10, 2–11 (2012).
Hollister, S. J. & Murphy, W. L. Scaffold translation: barriers between concept and clinic. Tissue Eng. Part B Rev. 17, 459–474 (2011).
Quarto R et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 344, 385–386 (2001).
Marcacci, M. et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 13, 947–955 (2007).
Zhang, Z. Y. et al. Neo-vascularization and bone formation mediated by fetal mesenchymal stem cell tissue-engineered bone grafts in critical-size femoral defects. Biomaterials 31, 608–620 (2010).
Wang, L. et al. Osteogenesis and angiogenesis of tissue-engineered bone constructed by prevascularized β-tricalcium phosphate scaffold and mesenchymal stem cells. Biomaterials 31, 9452–9461 (2010).
Zhao, M. et al. Repair of bone defect with vascularized tissue engineered bone graft seeded with mesenchymal stem cells in rabbits. Microsurgery 31, 130–137 (2011).
Choi, H. J. et al. Establishment of efficacy and safety assessment of human adipose tissue-derived mesenchymal stem cells (hATMSCs) in a nude rat femoral segmental defect model. J. Korean Med. Sci. 26, 482–491 (2011).
Diab, T. et al. A silk hydrogel-based delivery system of bone morphogenetic protein for the treatment of large bone defects. J. Mech. Behav. Biomed. Mater. 11, 123–131 (2012).
Fan, Z. X. et al. Placenta- versus bone-marrow-derived mesenchymal cells for the repair of segmental bone defects in a rabbit model. FEBS J. 279, 2455–2465 (2012).
Hou, J. et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed. Mater. 7, 035002 (2012).
Cao, L. et al. Experimental repair of segmental bone defects in rabbits by angiopoietin-1 gene transfected MSCs seeded on porous β-TCP scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 100, 1229–1236 (2012).
Bagher Z., Rajaei, F. & Shokrgozar, M. Comparative study of bone repair using porous hydroxyapatite/β-tricalcium phosphate and xenograft scaffold in rabbits with tibia defect. Iran Biomed. J. 16, 18–24 (2012).
De Guzman, R, C, et al. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 34, 1644–1656 (2013).
Rongen, J. J., van Tienen, T. G., van Bochove, B., Grijpma, D. W. & Buma, P. Biomaterials in search of a meniscus substitute. Biomaterials 35, 3527–3540 (2014).
Stone, K. R., Steadman, J. R., Rodkey, W. G. & Li, S. T. Regeneration of meniscal cartilage with use of a collagen scaffold. Analysis of preliminary data. J. Bone Joint Surg. Am. 79, 1770–1777 (1997).
Steadman, J. R. & Rodkey, W. G. Tissue-engineered collagen meniscus implants: 5- to 6-year feasibility study results. Arthroscopy 21, 515–525 (2005).
Rodkey, W. G. et al. Comparison of the collagen meniscus implant with partial meniscectomy. A prospective randomized trial. J. Bone Joint Surg. Am. 90, 1413–1426 (2008).
Bulgheroni, P. et al. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee 17, 224–229 (2010).
Zaffagnini, S. et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: a minimum 10-year follow-up study. Am. J. Sports Med. 39, 977–985 (2011).
Linke, R. D., Ulmer, M. &, Imhoff, A. B. Replacement of the meniscus with a collagen implant (CMI) [English, German]. Oper. Orthop. Traumatol. 18, 453–462 (2006).
Verdonk, R., Verdonk, P., Huysse, W., Forsyth, R. & Heinrichs, E. L. Tissue ingrowth after implantation of a novel, biodegradable polyurethane scaffold for treatment of partial meniscal lesions. Am. J. Sports Med. 39, 774–782 (2011).
Verdonk, P. et al. Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold: two-year safety and clinical outcomes. Am. J. Sports Med. 40, 844–853 (2012).
Bouyarmane, H. et al. Polyurethane scaffold in lateral meniscus segmental defects: clinical outcomes at 24 months follow-up. Orthop. Traumatol. Surg. Res. 100, 153–157 (2014).
Koning, M., Harmsen, M. C., Van luyn, M. J. & Werker, P. M. Current opportunities and challenges in skeletal muscle tissue engineering. J. Tissue Eng. Regen. Med. 3, 407–415 (2009).
Mertens, J. P., Sugg, K. B., Lee, J. D. & Larkin, L. M. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen. Med. 9, 89–100 (2014).
Cittadella Vigodarzere, G. & Mantero, S. Skeletal muscle tissue engineering: strategies for volumetric constructs. Front. Physiol. 5, 362 (2014).
Mase, V. J. Jr et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511 (2010).
Sicari, B. M. et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci. Transl. Med. 6, 234ra58 (2014).
Hoque, M. E., Chuan, Y. L. & Pashby, I. Extrusion based rapid prototyping technique: an advanced platform for tissue engineering scaffold fabrication. Biopolymers 97, 83–93 (2012).
Rengier, F. et al. 3D printing based on imaging data: review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 5, 335–341 (2010).
Ma, B., Xie, J., Jiang, J., Shuler, F. D. & Bartlett, D. E. Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine (Lond.) 8, 1459–1481 (2013).
Maude, S., Ingham, E. & Aggeli, A. Biomimetic self-assembling peptides as scaffolds for soft tissue engineering. Nanomedicine (Lond.) 8, 823–847 (2013).
Holzapfel, B. M. et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 65, 581–603 (2013).
Chen, W. et al. Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement-chitosan-RGD scaffold for bone repair. Tissue Eng. Part A 19, 915–927 (2013).
Chaisri, P., Chingsungnoen, A. & Siri, S. Repetitive Gly-Leu-Lys-Gly-Glu-Asn-Arg-Gly-Asp peptide derived from collagen and fibronectin for improving cell-scaffold interaction. Appl. Biochem. Biotechnol. http://dx.doi.org/10.1007/s12010-014-1388-y (2014).
Bertassoni, L. E. et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14, 2202–2211 (2014).
Sakaguchi, K. et al. In vitro engineering of vascularized tissue surrogates. Sci. Rep. 3, 1316 (2013).
Sekine, H. et al. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 4, 1399 (2013).
Eweida, A. M. et al. Enhancing mandibular bone regeneration and perfusion via axial vascularization of scaffolds. Clin. Oral Investig. 18, 1671–1678 (2014).
Kokemüller, H. et al. En bloc prefabrication of vascularized bioartificial bone grafts in sheep and complete workflow for custom-made transplants. Int. J. Oral Maxillofac. Surg. 43, 163–172 (2014).
Kosuge, D., Khan, W. S., Haddad, B. & Marsh, D. Biomaterials and scaffolds in bone and musculoskeletal engineering. Curr. Stem Cell Res. Ther. 8, 185–191 (2013).
Rowland, C. R., Little, D. & Guilak, F. Factors influencing the long-term behavior of extracellular matrix-derived scaffolds for musculoskeletal soft tissue repair. J. Long Term Eff. Med. Implants 22, 181–193 (2012).
Turner, N. J. & Badylak, S. F. Biologic scaffolds for musculotendinous tissue repair. Eur. Cell. Mater. 25, 130–143 (2013).
Chan, B. P. & Leong, K. W. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur. Spine J. 17 (Suppl. 4), 467–479 (2008).
O'Brien, F. J. Biomaterials & scaffolds for tissue engineering. Materials Today 14, 88–95 (2011).
Polo-Corrales, L., Latorre-Esteves, M. & Ramirez-Vick, J. E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 14, 15–56 (2014).
Tevlin, R. et al. Biomaterials for craniofacial bone engineering. J. Dent. Res. 93, 1187–1195 (2014).
Bobe, K. et al. In vitro and in vivo evaluation of biodegradable, open-porous scaffolds made of sintered magnesium W4 short fibres. Acta Biomater. 9, 8611–8623 (2013).
Chou, D. T. et al. Novel processing of iron-manganese alloy-based biomaterials by inkjet 3-D printing. Acta Biomater. 9, 8593–8603 (2013).
Matassi, F., Botti, A., Sirleo, L., Carulli, C. & Innocenti, M. Porous metal for orthopedics implants. Clin. Cases Miner. Bone Metab. 10, 111–115 (2013).
Brauker, J. H. et al. Neovascularization of synthetic membranes directed by membrane microarchitecture. J. Biomed. Mater. Res. 29, 1517–1524 (1995).
Klawitter, J. & Hulbert, S. F. Application of porous ceramics for the attachment of load-bearing internal orthopedic applications. J. Biomed. Mater. Res. 5, 161–229 (1971).
Whang, K et al. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 5, 35–51 (1999).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to researching data for the article, providing a substantial contribution to discussions of the content, writing the article, and to the review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Smith, B., Grande, D. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol 11, 213–222 (2015). https://doi.org/10.1038/nrrheum.2015.27
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.27
This article is cited by
-
Biologic Augmentation of Isolated Meniscal Repair
Current Reviews in Musculoskeletal Medicine (2024)
-
Meniscus repair: up-to-date advances in stem cell-based therapy
Stem Cell Research & Therapy (2022)
-
Biodegradable polymer nanocomposites for ligament/tendon tissue engineering
Journal of Nanobiotechnology (2020)
-
An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: focusing on cartilage tissue engineering
Journal of Biological Engineering (2019)
-
In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration
Nature Communications (2019)