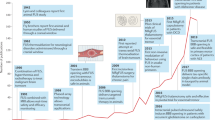
Key Points
-
Ultrasound is a mechanical pressure wave with a frequency above the range of human hearing (>20 kHz) that is increasingly used as a diagnostic, surgical, neuromodulation and drug delivery tool
-
Surgical ultrasound initially required craniotomies, but large phased ultrasound arrays have enabled clinical trials of surgical ultrasound through the skull for essential tremor, Parkinson disease, obsessive–compulsive disorder and chronic pain
-
In addition to surgery and neuromodulation, ultrasound has huge potential in its capacity to open the blood–brain barrier to allow drug delivery to the brain
-
Ultrasound therapy has shown promise in the treatment of Alzheimer disease; the unified biochemical basis of neurodegenerative diseases suggests that ultrasound could be effective in more of these conditions
-
Safety issues and attenuation of ultrasound by the human skull pose major challenges for the translation of ultrasound-based strategies for the prevention and treatment of neurological diseases
-
The combination of ultrasound technology and drug development has the potential to reduce, halt or even prevent neurodegenerative diseases and other brain disorders
Abstract
Like cardiovascular disease and cancer, neurological disorders present an increasing challenge for an ageing population. Whereas nonpharmacological procedures are routine for eliminating cancer tissue or opening a blocked artery, the focus in neurological disease remains on pharmacological interventions. Setbacks in clinical trials and the obstacle of access to the brain for drug delivery and surgery have highlighted the potential for therapeutic use of ultrasound in neurological diseases, and the technology has proved useful for inducing focused lesions, clearing protein aggregates, facilitating drug uptake, and modulating neuronal function. In this Review, we discuss milestones in the development of therapeutic ultrasound, from the first steps in the 1950s to recent improvements in technology. We provide an overview of the principles of diagnostic and therapeutic ultrasound, for surgery and transient opening of the blood–brain barrier, and its application in clinical trials of stroke, Parkinson disease and chronic pain. We discuss the promising outcomes of safety and feasibility studies in preclinical models, including rodents, pigs and macaques, and efficacy studies in models of Alzheimer disease. We also consider the challenges faced on the road to clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vykhodtseva, N., Sorrentino, V., Jolesz, F. A., Bronson, R. T. & Hynynen, K. MRI detection of the thermal effects of focused ultrasound on the brain. Ultrasound Med. Biol. 26, 871–880 (2000).
Arvanitis, C. D. & McDannold, N. Integrated ultrasound and magnetic resonance imaging for simultaneous temperature and cavitation monitoring during focused ultrasound therapies. Med. Phys. 40, 112901 (2013).
Fry, W. J., Mosberg, W. H. Jr, Barnard, J. W. & Fry, F. J. Production of focal destructive lesions in the central nervous system with ultrasound. J. Neurosurg. 11, 471–478 (1954).
Hynynen, K. et al. Pre-clinical testing of a phased array ultrasound system for MRI-guided noninvasive surgery of the brain — a primate study. Eur. J. Radiol. 59, 149–156 (2006).
Pernot, M. et al. In vivo transcranial brain surgery with an ultrasonic time reversal mirror. J. Neurosurg. 106, 1061–1066 (2007).
Bauer, R. et al. Noninvasive functional neurosurgery using transcranial MR imaging-guided focused ultrasound. Parkinsonism Relat. Disord. 20, S197–S199 (2014).
Chauvet, D. et al. Targeting accuracy of transcranial magnetic resonance-guided high-intensity focused ultrasound brain therapy: a fresh cadaver model. J. Neurosurg. 118, 1046–1052 (2013).
Louis, E. D. & Ferreira, J. J. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov. Disord. 25, 534–541 (2010).
Hanson, T. L., Fuller, A. M., Lebedev, M. A., Turner, D. A. & Nicolelis, M. A. Subcortical neuronal ensembles: an analysis of motor task association, tremor, oscillations, and synchrony in human patients. J. Neurosci. 32, 8620–8632 (2012).
Picillo, M. & Fasano, A. Recent advances in essential tremor: surgical treatment. Parkinsonism Relat. Disord. 22, S171–S175 (2016).
Lipsman, N. et al. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 12, 462–468 (2013).
Elias, W. J. et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 369, 640–648 (2013).
Magara, A. et al. First experience with MR-guided focused ultrasound in the treatment of Parkinson's disease. J. Ther. Ultrasound 2, 11 (2014).
Na, Y. C., Chang, W. S., Jung, H. H., Kweon, E. J. & Chang, J. W. Unilateral magnetic resonance-guided focused ultrasound pallidotomy for Parkinson disease. Neurology 85, 549–551 (2015).
Cetas, J. S., Saedi, T. & Burchiel, K. J. Destructive procedures for the treatment of nonmalignant pain: a structured literature review. J. Neurosurg. 109, 389–404 (2008).
Ram, Z. et al. Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 59, 949–955 (2006).
Jeanmonod, D. et al. Transcranial magnetic resonance imaging-guided focused ultrasound: noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 32, E1 (2012).
McDannold, N., Clement, G. T., Black, P., Jolesz, F. & Hynynen, K. Transcranial magnetic resonance imaging-guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 66, 323–332 (2010).
Medel, R. et al. Magnetic resonance-guided focused ultrasound surgery: part 2: a review of current and future applications. Neurosurgery 71, 755–763 (2012).
Coluccia, D. et al. First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J. Ther. Ultrasound 2, 17 (2014).
Barreto, A. D. et al. Safety and dose-escalation study design of Transcranial Ultrasound in Clinical SONolysis for acute ischemic stroke: the TUCSON Trial. Int. J. Stroke 4, 42–48 (2009).
Barreto, A. D. et al. CLOTBUST-Hands Free: pilot safety study of a novel operator-independent ultrasound device in patients with acute ischemic stroke. Stroke 44, 3376–3381 (2013).
Saqqur, M. et al. The role of sonolysis and sonothrombolysis in acute ischemic stroke: a systematic review and meta-analysis of randomized controlled trials and case–control studies. J. Neuroimaging 24, 209–220 (2014).
Daffertshofer, M. et al. Transcranial low-frequency ultrasound-mediated thrombolysis in brain ischemia: increased risk of hemorrhage with combined ultrasound and tissue plasminogen activator: results of a phase II clinical trial. Stroke 36, 1441–1446 (2005).
Baron, C., Aubry, J. F., Tanter, M., Meairs, S. & Fink, M. Simulation of intracranial acoustic fields in clinical trials of sonothrombolysis. Ultrasound Med. Biol. 35, 1148–1158 (2009).
Monteith, S. J. et al. Minimally invasive treatment of intracerebral hemorrhage with magnetic resonance-guided focused ultrasound. J. Neurosurg. 118, 1035–1045 (2013).
Lindstrom, P. A. Prefrontal ultrasonic irradiation — a substitute for lobotomy. AMA Arch. Neurol. Psychiatry 72, 399–425 (1954).
Nuttin, B. et al. Consensus on guidelines for stereotactic neurosurgery for psychiatric disorders. J. Neurol. Neurosurg. Psychiatry 85, 1003–1008 (2014).
Jung, H. H. et al. Bilateral thermal capsulotomy with MR-guided focused ultrasound for patients with treatment-refractory obsessive–compulsive disorder: a proof-of-concept study. Mol. Psychiatry 20, 1205–1211 (2014).
Stern, L. & Rothlin, E. Effets de l'action directe du curare sur les différentes parties du cervelet. Schweiz. Arch. Neurol. Psychiatr. 3, 234–254 (in French) (1918).
Coisne, C. & Engelhardt, B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid. Redox Signal. 15, 1285–1303 (2011).
Tietz, S. & Engelhardt, B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 209, 493–506 (2015).
Bauer, H. C., Krizbai, I. A., Bauer, H. & Traweger, A. “You shall not pass” — tight junctions of the blood brain barrier. Front. Neurosci. 8, 392 (2014).
Nag, S. Morphology and molecular properties of cellular components of normal cerebral vessels. Methods Mol. Med. 89, 3–36 (2003).
Wong, A. D. et al. The blood–brain barrier: an engineering perspective. Front. Neuroeng. 6, 7 (2013).
Pardridge, W. M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 32, 1959–1972 (2012).
Levites, Y. et al. Insights into the mechanisms of action of anti-Aβ antibodies in Alzheimer's disease mouse models. FASEB J. 20, 2576–2578 (2006).
Krueger, M., Hartig, W., Reichenbach, A., Bechmann, I. & Michalski, D. Blood–brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS ONE 8, e56419 (2013).
Knowland, D. et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood–brain barrier breakdown in stroke. Neuron 82, 603–617 (2014).
Gilad, R., Lampl, Y., Eilam, A., Boaz, M. & Loyberboim, M. SPECT–DTPA as a tool for evaluating the blood–brain barrier in post-stroke seizures. J. Neurol. 259, 2041–2044 (2012).
Mehta, D. C., Short, J. L. & Nicolazzo, J. A. Altered brain uptake of therapeutics in a triple transgenic mouse model of Alzheimer's disease. Pharm. Res. 30, 2868–2879 (2013).
Hawkes, C. A. et al. Regional differences in the morphological and functional effects of aging on cerebral basement membranes and perivascular drainage of amyloid-β from the mouse brain. Aging Cell 12, 224–236 (2013).
Bien-Ly, N. et al. Lack of widespread BBB disruption in Alzheimer's disease models: focus on therapeutic antibodies. Neuron 88, 289–297 (2015).
Sirsi, S. & Borden, M. Microbubble compositions, properties and biomedical applications. Bubble Sci. Eng. Technol. 1, 3–17 (2009).
Gateau, J. et al. In vivo bubble nucleation probability in sheep brain tissue. Phys. Med. Biol. 56, 7001–7015 (2011).
Leinenga, G. & Gotz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Sci. Transl. Med. 7, 278ra33 (2015).
Konofagou, E. E. Optimization of the ultrasound-induced blood–brain barrier opening. Theranostics 2, 1223–1237 (2012).
Appis, A. W., Tracy, M. J. & Feinstein, S. B. Update on the safety and efficacy of commercial ultrasound contrast agents in cardiac applications. Echo Res. Pract. 2, R55–R62 (2015).
Wrenn, S. P. et al. Bursting bubbles and bilayers. Theranostics 2, 1140–1159 (2012).
Fan, C. H. et al. Submicron-bubble-enhanced focused ultrasound for blood–brain barrier disruption and improved CNS drug delivery. PLoS ONE 9, e96327 (2014).
McDannold, N., Vykhodtseva, N. & Hynynen, K. Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys. Med. Biol. 51, 793–807 (2006).
Raymond, S. B., Skoch, J., Hynynen, K. & Bacskai, B. J. Multiphoton imaging of ultrasound/Optison mediated cerebrovascular effects in vivo. J. Cereb. Blood Flow Metab. 27, 393–403 (2007).
Caskey, C. F., Stieger, S. M., Qin, S., Dayton, P. A. & Ferrara, K. W. Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall. J. Acoust. Soc. Am. 122, 1191–1200 (2007).
Sheikov, N., McDannold, N., Sharma, S. & Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 34, 1093–1104 (2008).
O'Reilly, M. A. & Hynynen, K. Blood–brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology 263, 96–106 (2012).
Marty, B. et al. Dynamic study of blood–brain barrier closure after its disruption using ultrasound: a quantitative analysis. J. Cereb. Blood Flow Metab. 32, 1948–1958 (2012).
Baseri, B. et al. Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood–brain barrier using focused ultrasound and microbubbles. Phys. Med. Biol. 57, N65–N81 (2012).
Baseri, B., Choi, J. J., Tung, Y. S. & Konofagou, E. E. Multi-modality safety assessment of blood–brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med. Biol. 36, 1445–1459 (2010).
Choi, J. J. et al. Noninvasive and localized blood–brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J. Cereb. Blood Flow Metab. 31, 725–737 (2011).
Choi, J. J. et al. Microbubble-size dependence of focused ultrasound-induced blood–brain barrier opening in mice in vivo. IEEE Trans. Biomed. Eng. 57, 145–154 (2010).
Arvanitis, C. D., Livingstone, M. S., Vykhodtseva, N. & McDannold, N. Controlled ultrasound-induced blood–brain barrier disruption using passive acoustic emissions monitoring. PLoS ONE 7, e45783 (2012).
Liu, H. L. et al. Design and experimental evaluation of a 256-channel dual-frequency ultrasound phased-array system for transcranial blood–brain barrier opening and brain drug delivery. IEEE Trans. Biomed. Eng. 61, 1350–1360 (2014).
Choi, J. J., Selert, K., Vlachos, F., Wong, A. & Konofagou, E. E. Noninvasive and localized neuronal delivery using short ultrasonic pulses and microbubbles. Proc. Natl Acad. Sci. USA 108, 16539–16544 (2011).
McDannold, N., Vykhodtseva, N. & Hynynen, K. Blood–brain barrier disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med. Biol. 34, 834–840 (2008).
McDannold, N., Vykhodtseva, N. & Hynynen, K. Effects of acoustic parameters and ultrasound contrast agent dose on focused-ultrasound induced blood–brain barrier disruption. Ultrasound Med. Biol. 34, 930–937 (2008).
Beccaria, K. et al. Opening of the blood–brain barrier with an unfocused ultrasound device in rabbits. J. Neurosurg. 119, 887–898 (2013).
McDannold, N., Arvanitis, C. D., Vykhodtseva, N. & Livingstone, M. S. Temporary disruption of the blood–brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 72, 3652–3663 (2012).
Downs, M. E. et al. Long-term safety of repeated blood–brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE 10, e0125911 (2015).
Choi, J. J., Pernot, M., Small, S. A. & Konofagou, E. E. Noninvasive, transcranial and localized opening of the blood–brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 33, 95–104 (2007).
Choi, J. J., Pernot, M., Brown, T. R., Small, S. A. & Konofagou, E. E. Spatio-temporal analysis of molecular delivery through the blood–brain barrier using focused ultrasound. Phys. Med. Biol. 52, 5509–5530 (2007).
Kinoshita, M., McDannold, N., Jolesz, F. A. & Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl Acad. Sci. USA 103, 11719–11723 (2006).
Raymond, S. B. et al. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. PLoS ONE 3, e2175 (2008).
Etame, A. B. et al. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine 8, 1133–1142 (2012).
Mead, B. P. et al. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J. Control. Release 223, 109–117 (2015).
Reth, M. Matching cellular dimensions with molecular sizes. Nat. Immunol. 14, 765–767 (2013).
Hynynen, K., McDannold, N., Sheikov, N. A., Jolesz, F. A. & Vykhodtseva, N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. NeuroImage 24, 12–20 (2005).
Chen, H. & Konofagou, E. E. The size of blood–brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood Flow Metab. 34, 1197–1204 (2014).
Thevenot, E. et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 23, 1144–1155 (2012).
Wang, S., Olumolade, O. O., Sun, T., Samiotaki, G. & Konofagou, E. E. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 22, 104–110 (2015).
Winkler, E. A., Bell, R. D. & Zlokovic, B. V. Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405 (2011).
Choi, J. J. et al. Noninvasive and transient blood–brain barrier opening in the hippocampus of Alzheimer's double transgenic mice using focused ultrasound. Ultrason. Imaging 30, 189–200 (2008).
Burgess, A., Nhan, T., Moffatt, C., Klibanov, A. L. & Hynynen, K. Analysis of focused ultrasound-induced blood–brain barrier permeability in a mouse model of Alzheimer's disease using two-photon microscopy. J. Control. Release 192, 243–248 (2014).
Murugesan, N., Demarest, T. G., Madri, J. A. & Pachter, J. S. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol. Aging 33, 1004.e1–1004.e16 (2012).
Lucke-Wold, B. P., Logsdon, A. F., Turner, R. C., Rosen, C. L. & Huber, J. D. Aging, the metabolic syndrome, and ischemic stroke: redefining the approach for studying the blood–brain barrier in a complex neurological disease. Adv. Pharmacol. 71, 411–449 (2014).
Scarcelli, T. et al. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 7, 304–307 (2014).
Li, B. et al. Failure of neuronal maturation in Alzheimer disease dentate gyrus. J. Neuropathol. Exp. Neurol. 67, 78–84 (2008).
Tufail, Y. et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 66, 681–694 (2010).
Reher, P., Doan, N., Bradnock, B., Meghji, S. & Harris, M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine 11, 416–423 (1999).
Lin, W. T., Chen, R. C., Lu, W. W., Liu, S. H. & Yang, F. Y. Protective effects of low-intensity pulsed ultrasound on aluminium-induced cerebral damage in Alzheimer's disease rat model. Sci. Rep. 5, 9671 (2015).
Jordao, J. F. et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-β plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS ONE 5, e10549 (2010).
Jordao, J. F. et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 248, 16–29 (2013).
Burgess, A. et al. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood–brain barrier and improves pathologic abnormalities and behavior. Radiology 273, 736–745 (2014).
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell 142, 387–397 (2010).
Yamamoto, K., Shimada, H., Koh, H., Ataka, S. & Miki, T. Serum levels of albumin-amyloid β complexes are decreased in Alzheimer's disease. Geriatr. Gerontol. Int. 14, 716–723 (2014).
Dickson, D. W. Neuropathology of non-Alzheimer degenerative disorders. Int. J. Clin. Exp. Pathol. 3, 1–23 (2009).
US National Library of Science. ClinicalTrials.gov[online], (2015).
US National Library of Science. ClinicalTrials.gov[online], (2016).
Jun, S. B. Ultrasound as a noninvasive neuromodulation tool. Biomed. Eng. Lett. 2, 8–12 (2012).
Tyler, W. J. Noninvasive neuromodulation with ultrasound? A continuum mechanics hypothesis. Neuroscientist 17, 25–36 (2011).
Harvey, E. N. The effect of high frequency sound waves on heart muscle and other irritable tissues. Am. J. Physiol. 91, 284–290 (1929).
Juan, E. J., Gonzalez, R., Albors, G., Ward, M. P. & Irazoqui, P. Vagus nerve modulation using focused pulsed ultrasound: potential applications and preliminary observations in a rat. Int. J. Imaging Syst. Technol. 24, 67–71 (2014).
Krasovitski, B., Frenkel, V., Shoham, S. & Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl Acad. Sci. USA 108, 3258–3263 (2011).
Ibsen, S., Tong, A., Schutt, C., Esener, S. & Chalasani, S. H. Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat. Commun. 6, 8264 (2015).
McDannold, N. et al. Targeted, noninvasive blockade of cortical neuronal activity. Sci. Rep. 5, 16253 (2015).
Tsai, S. J. Transcranial focused ultrasound as a possible treatment for major depression. Med. Hypotheses 84, 381–383 (2015).
Fernandes, B. S. et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med. 13, 289 (2015).
Fry, F. J. Transkull transmission of an intense focused ultrasonic beam. Ultrasound Med. Biol. 3, 179–184 (1977).
Marsac, L. et al. MR-guided adaptive focusing of therapeutic ultrasound beams in the human head. Med. Phys. 39, 1141–1149 (2012).
Deffieux, T. & Konofagou, E. E. Numerical study of a simple transcranial focused ultrasound system applied to blood–brain barrier opening. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 57, 2637–2653 (2010).
Martin, E., Jeanmonod, D., Morel, A., Zadicario, E. & Werner, B. High-intensity focused ultrasound for noninvasive functional neurosurgery. Ann. Neurol. 66, 858–861 (2009).
Aubry, J. F. & Tanter, M. MR-Guided transcranial focused ultrasound. Adv. Exp. Med. Biol. 880, 97–111 (2016).
Kyriakou, A. et al. A review of numerical and experimental compensation techniques for skull-induced phase aberrations in transcranial focused ultrasound. Int. J. Hyperthermia 30, 36–46 (2014).
Tufail, Y., Yoshihiro, A., Pati, S., Li, M. M. & Tyler, W. J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 6, 1453–1470 (2011).
Min, B. K. et al. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 12, 23 (2011).
Younan, Y. et al. Influence of the pressure field distribution in transcranial ultrasonic neurostimulation. Med. Phys. 40, 082902 (2013).
Deffieux, T. et al. Low-intensity focused ultrasound modulates monkey visuomotor behavior. Curr. Biol. 23, 2430–2433 (2013).
Lee, W. et al. Image-guided focused ultrasound-mediated regional brain stimulation in sheep. Ultrasound Med. Biol. 42, 459–470 (2016).
Legon, W. et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 17, 322–329 (2014).
George, P. M. & Steinberg, G. K. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 87, 297–309 (2015).
Theismann, H. & Pfander, F. The permeability of bone for ultrasound. Strahlentherapie 80, 607–610 (in German) (1949).
Hueter, H. F. Messung der Ultraschallabsorption im menschlichen Schädelknochen und ihre Abhängigkeit von der Frequenz. Naturwissenschaften 39, 21–22 (in German) (1952).
White, D. N., Curry, G. R. & Stevenson, R. J. The acoustic characteristics of the skull. Ultrasound Med. Biol. 4, 225–252 (1978).
Fry, F. J. & Barger, J. E. Acoustical properties of the human skull. J. Acoust. Soc. Am. 63, 1576–1590 (1978).
Phillips, D. J., Smith, S. W., von Ramm, O. T. & Thurnstone, F. L. in Acoustical Holography 1st edn Vol. 6 Ch. 6 (ed. Booth, N.) 103–120 (Springer Science + Business Media, 1975).
Hynynen, K. & Sun, J. Trans-skull ultrasound therapy: the feasibility of using image-derived skull thickness information to correct the phase distortion. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 46, 752–755 (1999).
O'Reilly, M. A. & Hynynen, K. A super-resolution ultrasound method for brain vascular mapping. Med. Phys. 40, 110701 (2013).
Ittner, L. M. & Götz, J. Amyloid-β and tau — a toxic pas de deux in Alzheimer's disease. Nat. Rev. Neurosci. 12, 65–72 (2011).
Zhu, C. W. & Sano, M. Economic considerations in the management of Alzheimer's disease. Clin. Interv. Aging 1, 143–154 (2006).
Golde, T. E. Open questions for Alzheimer's disease immunotherapy. Alzheimers Res. Ther. 6, 3 (2014).
Wu, S. Y. et al. Transcranial cavitation detection in primates during blood–brain barrier opening — a performance assessment study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 61, 966–978 (2014).
Gateau, J., Aubry, J. F., Pernot, M., Fink, M. & Tanter, M. Combined passive detection and ultrafast active imaging of cavitation events induced by short pulses of high-intensity ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 58, 517–532 (2011).
Miller, G. W., Eames, M., Snell, J. & Aubry, J. F. Ultrashort echo-time MRI versus CT for skull aberration correction in MR-guided transcranial focused ultrasound: in vitro comparison on human calvaria. Med. Phys. 42, 2223–2233 (2015).
Schweinhardt, P. & Bushnell, M. C. Pain imaging in health and disease — how far have we come? J. Clin. Invest. 120, 3788–3797 (2010).
Acknowledgements
The authors' work is supported by the Estate of Dr Clem Jones AO and by grants from the Australian Research Council (DP130101932), the National Health and Medical Research Council of Australia (APP1037746, APP1003150) and the Australian Research Council Linkage Infrastructure, Equipment and Facilities scheme (LE100100074). We thank Matt Pelekanos for suggesting the term 'obicodilation', and Rowan Tweedale for critically reading the manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and made substantial contributions to discussion of the content. J.G., G.L. and C.L. wrote the manuscript. J.G., G.L. and R.N. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Leinenga, G., Langton, C., Nisbet, R. et al. Ultrasound treatment of neurological diseases — current and emerging applications. Nat Rev Neurol 12, 161–174 (2016). https://doi.org/10.1038/nrneurol.2016.13
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2016.13
This article is cited by
-
Mechanosensitive channel of large conductance enhances the mechanical stretching-induced upregulation of glycolysis and oxidative metabolism in Schwann cells
Cell Communication and Signaling (2024)
-
Recent advances in the molecular mechanisms of low-intensity pulsed ultrasound against inflammation
Journal of Molecular Medicine (2023)
-
Ultrasound-triggered microbubble destruction enhances the radiosensitivity of glioblastoma by inhibiting PGRMC1-mediated autophagy in vitro and in vivo
Military Medical Research (2022)
-
Characterization of passive permeability after low intensity focused ultrasound mediated blood–brain barrier disruption in a preclinical model
Fluids and Barriers of the CNS (2022)
-
Anti-Parkinsonian Therapy: Strategies for Crossing the Blood–Brain Barrier and Nano-Biological Effects of Nanomaterials
Nano-Micro Letters (2022)