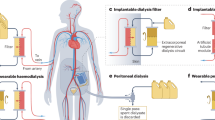
Abstract
Functional deterioration of the peritoneal membrane in patients on peritoneal dialysis has been described as being the result of a combination of neoangiogenesis and fibrosis. Glucose, glucose degradation products, and the unphysiological pH of the dialysate solution contribute to these changes. Although newer solutions clearly perform better in terms of their biocompatibility in an in vitro setting and in animal models, the benefit of such solutions over older solutions in the clinical setting is so far unproven. The difficulties in showing a benefit of the newer, more biocompatible solutions in the clinical setting can be explained by the fact that other factors also affect the properties of the peritoneal membrane. These factors are often neglected in clinical studies, which results in unnoticed differences in case-mix and blurs the potential impact of the novel solutions. However, many of these factors are modifiable, and attention should be paid to them in clinical practice to maintain the integrity of the peritoneal membrane. This Review focuses on factors that potentially influence the integrity of the peritoneal membrane, other than those associated with the peritoneal dialysis fluid itself.
Key Points
-
Factors other than those related to the peritoneal dialysis fluid can have important effects on the peritoneal membrane, and can result in variability in peritoneal function
-
The degree of glycaemic control rather than diabetes per se can cause changes to the peritoneal membrane; studies should therefore use haemoglobin A1c level instead of the dichotomized classification of diabetes/no diabetes
-
A high salt intake can induce changes in the peritoneal membrane by inducing hypertonic exchanges and by directly inducing peritoneal membrane changes
-
Peritoneal membrane characteristics can be influenced by genetic polymorphisms; further exploration of genetic polymorphisms in patients with encapsulating peritoneal sclerosis are warranted
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lameire, N., Van Biesen, W. & Vanholder, R. The role of peritoneal dialysis as first modality in an integrative approach to patients with end-stage renal disease. Perit. Dial. Int. 20 (Suppl. 2), S134–S141 (2000).
Davies, S. J. et al. What really happens to people on long-term peritoneal dialysis? Kidney Int. 54, 2207–2217 (1998).
Williams, J. D. et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 13, 470–479 (2002).
Wieslander, A. P. Cytotoxicity of peritoneal dialysis fluid—is it related to glucose breakdown products? Nephrol. Dial. Transplant. 11, 958–959 (1996).
Fusshoeller, A., Baehr, J., Grabensee, B. & Plum, J. Biocompatibility of a bicarbonate/lactate-buffered PD fluid tested with a double-chamber cell culture system. Perit. Dial. Int. 25, 387–393 (2005).
Grossin, N. et al. Improved in vitro biocompatibility of bicarbonate-buffered peritoneal dialysis fluid. Perit. Dial. Int. 26, 664–670 (2006).
Oh, E. J. et al. Impact of low glucose degradation product bicarbonate/lactate-buffered dialysis solution on the epithelial-mesenchymal transition of peritoneum. Am. J. Nephrol. 31, 58–67 (2010).
Witowski, J. et al. Effect of glucose degradation products on human peritoneal mesothelial cell function. J. Am. Soc. Nephrol. 11, 729–739 (2000).
Witowski, J. et al. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J. Am. Soc. Nephrol. 12, 2434–2441 (2001).
Kim, C. D. et al. Effects of low glucose degradation products peritoneal dialysis fluid on the peritoneal fibrosis and vascularization in a chronic rat model. Ther. Apher. Dial. 11, 56–64 (2007).
Mortier, S., De Vriese, A. S. & Lameire, N. Recent concepts in the molecular biology of the peritoneal membrane—implications for more biocompatible dialysis solutions. Blood Purif. 21, 14–23 (2003).
Mortier, S., Faict, D., Schalkwijk, C. G., Lameire, N. H. & De Vriese, A. S. Long-term exposure to new peritoneal dialysis solutions: effects on the peritoneal membrane. Kidney Int. 66, 1257–1265 (2004).
Mortier, S., Faict, D., Lameire, N. H. & De Vriese, A. S. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int. 67, 1559–1565 (2005).
Mortier, S., Faict, D., Gericke, M., Lameire, N. & De Vriese, A. Effects of new peritoneal dialysis solutions on leukocyte recruitment in the rat peritoneal membrane. Nephron Exp. Nephrol. 101, e139–e145 (2005).
Witowski, J. & Jorres, A. Effects of peritoneal dialysis solutions on the peritoneal membrane: clinical consequences. Perit. Dial. Int. 25 (Suppl. 3), S31–S34 (2005).
Johnson, D. W. et al. Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes. J. Am. Soc. Nephrol. 23, 1097–1107 (2012).
Sampimon, D. E., Coester, A. M., Struijk, D. G. & Krediet, R. T. The time course of peritoneal transport parameters in peritoneal dialysis patients who develop encapsulating peritoneal sclerosis. Nephrol. Dial. Transplant. 26, 291–298 (2011).
Garcia-Lopez, E., Lindholm, B. & Davies, S. An update on peritoneal dialysis solutions. Nat. Rev. Nephrol. 8, 224–233 (2012).
Rumpsfeld, M., McDonald, S. P., Purdie, D. M., Collins, J. & Johnson, D. W. Predictors of baseline peritoneal transport status in Australian and New Zealand peritoneal dialysis patients. Am. J. Kidney Dis. 43, 492–501 (2004).
Van Biesen, W. et al. The personal dialysis capacity test is superior to the peritoneal equilibration test to discriminate inflammation as the cause of fast transport status in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 1, 269–274 (2006).
Nakamoto, H. et al. Effect of diabetes on peritoneal function assessed by personal dialysis capacity test in patients undergoing CAPD. Am. J. Kidney Dis. 40, 1045–1054 (2002).
Honda, K. et al. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin. J. Am. Soc. Nephrol. 3, 720–728 (2008).
Stoenoiu, M. S. et al. Experimental diabetes induces functional and structural changes in the peritoneum. Kidney Int. 62, 668–678 (2002).
ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
De Vriese, A. S., Tilton, R. G., Stephan, C. C. & Lameire, N. H. Vascular endothelial growth factor is essential for hyperglycemia-induced structural and functional alterations of the peritoneal membrane. J. Am. Soc. Nephrol. 12, 1734–1741 (2001).
Combet, S. et al. Chronic uremia induces permeability changes, increased nitric oxide synthase expression, and structural modifications in the peritoneum. J. Am. Soc. Nephrol. 12, 2146–2157 (2001).
Vrtovsnik, F. et al. Induction of chronic kidney failure in a long-term peritoneal exposure model in the rat: effects on functional and structural peritoneal alterations. Perit. Dial. Int. 30, 558–569 (2010).
Kakuta, T. et al. Pyridoxamine improves functional, structural, and biochemical alterations of peritoneal membranes in uremic peritoneal dialysis rats. Kidney Int. 68, 1326–1336 (2005).
Osada, S. et al. Alterations in proteoglycan components and histopathology of the peritoneum in uraemic and peritoneal dialysis (PD) patients. Nephrol. Dial. Transplant. 24, 3504–3512 (2009).
De Vriese, A. S., Tilton, R. G., Mortier, S. & Lameire, N. H. Myofibroblast transdifferentiation of mesothelial cells is mediated by RAGE and contributes to peritoneal fibrosis in uraemia. Nephrol. Dial. Transplant. 21, 2549–2555 (2006).
Numata, M. et al. Possible pathologic involvement of receptor for advanced glycation end products (RAGE) for development of encapsulating peritoneal sclerosis in Japanese CAPD patients. Clin. Nephrol. 62, 455–460 (2004).
Pletinck, A. et al. Salt intake induces epithelial-to-mesenchymal transition of the peritoneal membrane in rats. Nephrol. Dial. Transplant. 25, 1688–1696 (2010).
Pecoits-Filho, R., Carvalho, M. J., Stenvinkel, P., Lindholm, B. & Heimburger, O. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit. Dial. Int. 26, 53–63 (2006).
Stenvinkel, P., Barany, P., Heimburger, O., Pecoits-Filho, R. & Lindholm, B. Mortality, malnutrition, and atherosclerosis in ESRD: what is the role of interleukin-6? Kidney Int. Suppl. 103–108 (2002).
Sawai, A. et al. Peritoneal macrophage infiltration is correlated with baseline peritoneal solute transport rate in peritoneal dialysis patients. Nephrol. Dial. Transplant. 26, 2322–2332 (2011).
Oh, K. H. et al. Intra-peritoneal interleukin-6 system is a potent determinant of the baseline peritoneal solute transport in incident peritoneal dialysis patients. Nephrol. Dial. Transplant. 25, 1639–1646 (2010).
Gillerot, G. et al. Genetic and clinical factors influence the baseline permeability of the peritoneal membrane. Kidney Int. 67, 2477–2487 (2005).
Hwang, Y. H. et al. Effects of interleukin-6 T15A single nucleotide polymorphism on baseline peritoneal solute transport rate in incident peritoneal dialysis patients. Perit. Dial. Int. 29, 81–88 (2009).
Oh, K. H. et al. Baseline peritoneal solute transport rate is not associated with markers of systemic inflammation or comorbidity in incident Korean peritoneal dialysis patients. Nephrol. Dial. Transplant. 23, 2356–2364 (2008).
Lee, Y. T. et al. Association between interleukin-10 gene polymorphism -592 (A/C) and peritoneal transport in patients undergoing peritoneal dialysis. Nephrology (Carlton) 16, 663–671 (2011).
Johnson, D. W. et al. Superior survival of high transporters treated with automated versus continuous ambulatory peritoneal dialysis. Nephrol. Dial. Transplant. 25, 1973–1979 (2010).
Rodrigues, A. S. et al. Evaluation of peritoneal transport and membrane status in peritoneal dialysis: focus on incident fast transporters. Am. J. Nephrol. 27, 84–91 (2007).
Wong, T. Y. et al. Association of ENOS polymorphism with basal peritoneal membrane function in uremic patients. Am. J. Kidney Dis. 42, 781–786 (2003).
Zeier, M. et al. Glucose degradation products in PD fluids: do they disappear from the peritoneal cavity and enter the systemic circulation? Kidney Int. 63, 298–305 (2003).
Thomas, M. C. Advanced glycation end products. Contrib. Nephrol. 170, 66–74 (2011).
Thornalley, P. J. & Rabbani, N. Highlights and hotspots of protein glycation in end-stage renal disease. Semin. Dial. 22, 400–404 (2009).
De Vriese, A. S., Flyvbjerg, A., Mortier, S., Tilton, R. G. & Lameire, N. H. Inhibition of the interaction of AGE–RAGE prevents hyperglycemia-induced fibrosis of the peritoneal membrane. J. Am. Soc. Nephrol. 14, 2109–2118 (2003).
Shu, K. H. et al. Association of interleukin-1β gene polymorphism and peritonitis in uremic patients undergoing peritoneal dialysis. Blood Purif. 32, 156–160 (2011).
Uchiyama, K. et al. Impact of a genetic polymorphism of the interleukin-1 receptor antagonist on technique survival in peritoneal dialysis patients. Blood Purif. 23, 450–458 (2005).
Bellon, T. et al. Alternative activation of macrophages in human peritoneum: implications for peritoneal fibrosis. Nephrol. Dial. Transplant. 26, 2995–3005 (2011).
Hagg, D. A. et al. Expression of chemokine (C-C motif) ligand 18 in human macrophages and atherosclerotic plaques. Atherosclerosis 204, e15–e20 (2009).
Sampimon, D. E., Vlijm, A., Phoa, S. S., Krediet, R. T. & Struijk, D. G. Encapsulating peritoneal sclerosis in a peritoneal dialysis patient using biocompatible fluids only: is Alport syndrome a risk factor? Perit. Dial. Int. 30, 240–242 (2010).
Nessim, S. J., Perl, J. & Bargman, J. M. The renin-angiotensin-aldosterone system in peritoneal dialysis: is what is good for the kidney also good for the peritoneum? Kidney Int. 78, 23–28 (2010).
Noh, H. et al. Angiotensin II mediates high glucose-induced TGF-β1 and fibronectin upregulation in HPMC through reactive oxygen species. Perit. Dial. Int. 25, 38–47 (2005).
Kiribayashi, K. et al. Angiotensin II induces fibronectin expression in human peritoneal mesothelial cells via ERK1/2 and p38 MAPK. Kidney Int. 67, 1126–1135 (2005).
Nakamoto, H. et al. Role of the renin-angiotensin system in the pathogenesis of peritoneal fibrosis. Perit. Dial. Int. 28 (Suppl. 3), S83–S87 (2008).
Mizuiri, S. et al. Effects of new peritoneal dialysis solutions, pyridoxamine and AT1 receptor blocker, on TGF-β1 and VEGF expression in rat peritoneal mesothelial cells. Am. J. Nephrol. 30, 295–302 (2009).
Duman, S. et al. Does enalapril prevent peritoneal fibrosis induced by hypertonic (3.86%) peritoneal dialysis solution? Perit. Dial. Int. 21, 219–224 (2001).
Kolesnyk, I., Struijk, D. G., Dekker, F. W. & Krediet, R. T. Effects of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in patients with chronic kidney disease. Neth. J. Med. 68, 15–23 (2010).
Jing, S., Kezhou, Y., Hong, Z., Qun, W. & Rong, W. Effect of renin-angiotensin system inhibitors on prevention of peritoneal fibrosis in peritoneal dialysis patients. Nephrology (Carlton) 15, 27–32 (2010).
Wontanatawatot, W., Eiam-Ong, S., Leelahavanichkul, A. & Kanjanabuch, T. An update on RAAS blockade and peritoneal membrane preservation: the ace of art. J. Med. Assoc. Thai. 94 (Suppl. 4), S175–S183 (2011).
Lewis, R. V. & McDevitt, D. G. Adverse reactions and interactions with beta-adrenoceptor blocking drugs. Med. Toxicol. 1, 343–361 (1986).
Oules, R., Challah, S. & Brunner, F. P. Case-control study to determine the cause of sclerosing peritoneal disease. Nephrol. Dial. Transplant. 3, 66–69 (1988).
Hendriks, P. M. et al. Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risk factors, and peritoneal transport kinetics. Perit. Dial. Int. 17, 136–143 (1997).
Stegmayr, B. G. Beta-blockers may cause ultrafiltration failure in peritoneal dialysis patients. Perit. Dial. Int. 17, 541–545 (1997).
Topley, N. et al. Human peritoneal mesothelial cell prostaglandin synthesis: induction of cyclooxygenase mRNA by peritoneal macrophage-derived cytokines. Kidney Int. 46, 900–909 (1994).
Liu, H. et al. A selective cyclooxygenase-2 inhibitor decreases transforming growth factor-beta1 synthesis and matrix production in human peritoneal mesothelial cells. Cell Biol. Int. 31, 508–515 (2007).
Douma, C. E., de Waart, D. R., Zemel, D., Struijk, D. G. & Krediet, R. T. Prostaglandin inhibition by intraperitoneal indomethacin has no effect on peritoneal permeability during stable CAPD. Nephrol. Dial. Transplant. 16, 803–808 (2001).
Aroeira, L. S. et al. Cyclooxygenase-2 mediates dialysate-induced alterations of the peritoneal membrane. J. Am. Soc. Nephrol. 20, 582–592 (2009).
Fabbrini, P. et al. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol. Dial. Transplant. 24, 3669–3676 (2009).
Polubinska, A. et al. Dialysis solution containing hyaluronan: effect on peritoneal permeability and inflammation in rats. Kidney Int. 57, 1182–1189 (2000).
Flessner, M. F. The role of extracellular matrix in transperitoneal transport of water and solutes. Perit. Dial. Int. 21 (Suppl. 3), S24–S29 (2001).
Harenberg, J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med. Res. Rev. 18, 1–20 (1998).
De Vriese, A. S., Mortier, S. & Lameire, N. H. Non anticoagulant effects of heparin: implications for animal models of peritoneal dialysis. Perit. Dial. Int. 21 (Suppl. 3), S354–S356 (2001).
Margetts, P. Heparin and the peritoneal membrane. Perit. Dial. Int. 29, 16–19 (2009).
Gambaro, G. et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N. A. S. randomized trial. J. Am. Soc. Nephrol. 13, 1615–1625 (2002).
Pletinck, A. et al. Oral supplementation with sulodexide inhibits neo-angiogenesis in a rat model of peritoneal perfusion. Nephrol. Dial. Transplant. 27, 548–556 (2012).
Fracasso, A. et al. Effect of oral treatment with the glycosaminoglycan sulodexide on peritoneal transport in CAPD patients. Perit. Dial. Int. 23, 595–599 (2003).
Horiuchi, T. et al. Image analysis of remesothelialization following chemical wounding of cultured human peritoneal mesothelial cells: the role of hyaluronan synthesis. Kidney Int. 64, 2280–2290 (2003).
Sitter, T., Sauter, M. & Haslinger, B. Modulation of fibrinolytic system components in mesothelial cells by hyaluronan. Perit. Dial. Int. 23, 222–227 (2003).
Szeto, C. C. et al. Dialysate hyaluronan concentration predicts survival but not peritoneal sclerosis in continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 36, 609–614 (2000).
Wang, T. et al. Hyaluronan decreases peritoneal fluid absorption: effect of molecular weight and concentration of hyaluronan. Kidney Int. 55, 667–673 (1999).
Wang, T. et al. Hyaluronan prevents the decreased net ultrafiltration caused by increased peritoneal dialysate fill volume. Kidney Int. 53, 496–502 (1998).
Wang, T. et al. Intraperitoneal addition of hyaluronan improves peritoneal dialysis efficiency. Perit. Dial. Int. 19 (Suppl. 2), S106–S111 (1999).
Moberly, J. B. et al. Effects of intraperitoneal hyaluronan on peritoneal fluid and solute transport in peritoneal dialysis patients. Perit. Dial. Int. 23, 63–73 (2003).
Rosengren, B. I., Carlsson, O. & Rippe, B. Hyaluronan and peritoneal ultrafiltration: a test of the “filter-cake” hypothesis. Am. J. Kidney Dis. 37, 1277–1285 (2001).
Wang, T. et al. Hyaluronan decreases peritoneal fluid absorption in peritoneal dialysis. J. Am. Soc. Nephrol. 8, 1915–1920 (1997).
Kihm, L. P. et al. Benfotiamine protects against peritoneal and kidney damage in peritoneal dialysis. J. Am. Soc. Nephrol. 22, 914–926 (2011).
Flessner, M. F. et al. Peritoneal changes after exposure to sterile solutions by catheter. J. Am. Soc. Nephrol. 18, 2294–2302 (2007).
Peters, T. et al. Mouse model of foreign body reaction that alters the submesothelium and transperitoneal transport. Am. J. Physiol. Renal Physiol. 300, F283–F289 (2011).
Author information
Authors and Affiliations
Contributions
A. Pletinck and W. Van Biesen researched data for the article, made a substantial contribution to discussion of content, wrote the article, and reviewed/edited the manuscript before submission. R. Vanholder and N. Veys made a substantial contribution to discussion of content and were involved in the review/editing of manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors' laboratory has received unrestricted grants from Baxter and Fresenius for basic scientific research. Wim Van Biesen has received honoraria from Baxter, Fresenius and Gambro. Raymond Vanholder has received honoraria from Fresenius and is a consultant for Baxter.
Rights and permissions
About this article
Cite this article
Pletinck, A., Vanholder, R., Veys, N. et al. Protecting the peritoneal membrane: factors beyond peritoneal dialysis solutions. Nat Rev Nephrol 8, 542–550 (2012). https://doi.org/10.1038/nrneph.2012.144
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.144
This article is cited by
-
IL-1β may be an indicator of peritoneal deterioration after healing of peritoneal dialysis-associated peritonitis
BMC Nephrology (2023)
-
Adipose-derived mesenchymal stem cells attenuate dialysis-induced peritoneal fibrosis by modulating macrophage polarization via interleukin-6
Stem Cell Research & Therapy (2021)
-
Expression of XBP1s in peritoneal mesothelial cells is critical for inflammation-induced peritoneal fibrosis
Scientific Reports (2019)
-
Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling
Cell Biology and Toxicology (2019)
-
CD147 expression in peritoneal injury
Clinical and Experimental Nephrology (2017)