Abstract
Peritoneal dialysis (PD) is an important home-based treatment for kidney failure and accounts for 11% of all dialysis and 9% of all kidney replacement therapy globally. Although PD is available in 81% of countries, this provision ranges from 96% in high-income countries to 32% in low-income countries. Compared with haemodialysis, PD has numerous potential advantages, including a simpler technique, greater feasibility of use in remote communities, generally lower cost, lesser need for trained staff, fewer management challenges during natural disasters, possibly better survival in the first few years, greater ability to travel, fewer dietary restrictions, better preservation of residual kidney function, greater treatment satisfaction, better quality of life, better outcomes following subsequent kidney transplantation, delayed need for vascular access (especially in small children), reduced need for erythropoiesis-stimulating agents, and lower risk of blood-borne virus infections and of SARS-CoV-2 infection. PD outcomes have been improving over time but with great variability, driven by individual and system-level inequities and by centre effects; this variation is exacerbated by a lack of standardized outcome definitions. Potential strategies for outcome improvement include enhanced standardization, monitoring and reporting of PD outcomes, and the implementation of continuous quality improvement programmes and of PD-specific interventions, such as incremental PD, the use of biocompatible PD solutions and remote PD monitoring.
Key points
-
Peritoneal dialysis (PD) has distinct advantages compared with haemodialysis, including the convenience of home treatment, improved quality of life, technical simplicity, lesser need for trained staff, greater cost-effectiveness in most countries, improved equity of access to dialysis in resource-limited settings, and improved survival, particularly in the first few years of initiating therapy.
-
Important barriers can hamper PD utilization in low-income settings, including the high costs of PD fluids (owing to the inability to manufacture them locally and the exorbitant costs of their import), limited workforce availability and a practice culture that limits optimal PD use, often leading to suboptimal outcomes.
-
PD outcomes are highly variable around the world owing in part to the use of variable outcome definitions, a heterogeneous practice culture, the lack of standardized monitoring and reporting of quality indicators, and kidney failure care gaps (including health care workforce shortages, inadequate health care financing, suboptimal governance and a lack of good health care information systems).
-
Key outcomes include not only clinical outcomes (typically defined as medical outcomes based on clinician assessment or diagnosis) — for example, PD-related infections, technique survival, mechanical complications, hospitalizations and PD-related mortality — but also patient-reported outcomes. These outcomes are directly reported by patients and focus on how they function or feel, typically in relation to quality of life or symptoms; patient-reported outcomes are used less frequently than clinical outcomes in day-to-day routine care.
Similar content being viewed by others
Introduction
Worldwide, peritoneal dialysis (PD) accounts for 9% of all kidney replacement therapy (KRT) and 11% of all dialysis1,2,3. According to the 2018 International Society of Nephrology Global Kidney Health Atlas (ISN-GKHA), the median global prevalence of PD was 38.1 per million population (pmp) but varied over 5,000-fold from 0.1 pmp in Egypt to 531 pmp in Hong Kong2. More than half of all patients receiving PD resided in four countries (China, USA, Mexico and Thailand)4. PD was not available in 30 countries, 20 of which were located in Africa. The survey further demonstrated that PD use was 60-fold lower in low-income countries (LICs; 0.9 pmp, 95% CI 0.7–1.5) than in high-income countries (HICs; 53.0 pmp, 95% CI 40.6–89.8 pmp)4. This observation seems initially somewhat surprising given that, compared with haemodialysis (HD), PD has a number of distinct advantages that should be attractive to LICs, such as greater technical simplicity, lesser need for trained staff and lower nurse-to-patient ratios, greater feasibility in rural and remote communities, fewer management challenges during natural disasters, greater cost-effectiveness (in most countries), improved equity of access to dialysis in resource-limited settings and possibly better survival in the first few years1,5,6,7,8. Indeed, because of these features of PD, a number of jurisdictions, such as Thailand, Hong Kong, mainland China, Australia, New Zealand and the USA, have implemented policies and/or financial incentives that favour the use of PD3,9,10,11,12. However, numerous barriers to PD utilization exist in many low- and middle-income countries (LMICs), including high PD fluid costs, lack of trained health care workforce, and variable but often poor outcomes (particularly related to infection)2,13.
Reported PD outcomes vary greatly around the world, partly owing to discrepancies in outcome definitions, practices, and monitoring and reporting of quality indicators, as well as kidney failure care gaps, including health care workforce shortages, inadequate health care financing, suboptimal governance, lack of suitable health care information systems and poor accessibility to kidney care3,4,14,15. These gaps are greatest in Africa and South Asia4.
In this Review, we describe the contemporary worldwide epidemiology of PD outcomes, including clinical, patient-reported and surrogate outcomes (Box 1), In particular, we will focus on prioritization and standardization of PD outcomes, comparisons with HD, potential mechanisms underlying PD outcomes, and strategies for outcome improvement, including PD-related interventions such incremental PD, the use of biocompatible PD solutions, remote PD monitoring, prescription changes and the use of assisted PD.
Standardizing and prioritizing PD outcomes
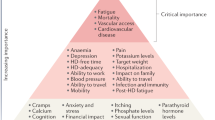
A major limitation to monitoring, reporting and benchmarking of outcomes in patients receiving PD has been the lack of use of standardized outcomes that are relevant and meaningful to patients and their caregivers. For example, PD-related infection is widely monitored and reported by PD units around the world because it is a major barrier to patients selecting PD as a dialysis modality, it is the commonest reason for patient transfer to HD and has a mortality of 2–6%16. However, in a systematic review of 120 randomized controlled trials (RCTs) in PD up to 2019, PD-related infection was reported in 59 (49%) studies using 383 different measures, of which 317 were only used once17. From 2016 to 2020, the Standardized Outcomes in Nephrology in PD (SONG-PD) initiative conducted a five-phase mixed methods process18, including systematic review, nominal group technique19, stakeholder interviews, an international Delphi survey20 and an international consensus workshop21, to identify the top five most important outcomes based on the shared priorities of patients, caregivers and health care professionals. These outcomes were, in descending order of priority, PD infection, cardiovascular disease (CVD), mortality, technique survival and life participation (Fig. 1). Notably, they differ somewhat from the core outcomes of critical importance identified for HD (fatigue, CVD, vascular access and mortality)22. In 2022, the International Society for Peritoneal Dialysis (ISPD) published its standardized definition for PD-related peritonitis, which should be diagnosed when at least two of the following are present: clinical features consistent with peritonitis (that is, abdominal pain and/or cloudy dialysis effluent); dialysis effluent white cell count >100/µl or >0.1 × 109/l (after a dwell time of at least 2 h), with >50% polymorphonuclear leukocytes; and positive dialysis effluent culture23. Work is currently underway to develop validated outcomes for technique survival and life participation24,25.
The Standardized Outcomes in Nephrology in Peritoneal Dialysis (SONG-PD) initiative identified a hierarchy of PD outcomes according to their level of importance to stakeholder groups. The outcomes in the top tier are critically important to all stakeholder groups, those in the middle tier are critically important to some stakeholder groups and those in the bottom tier are important to some or all stakeholder groups. Adapted with permission from ref.21, Elsevier.
Clinical PD outcomes across the globe
Clinical outcomes are medical outcomes based on clinician assessment or diagnosis. In this section, we discuss the clinical outcomes of infection, mortality, CVD, technique survival, hospitalization, encapsulating peritoneal sclerosis, mechanical complications and cognitive function.
PD-related infection
PD-related infection was rated as the most critically important outcome by PD patients, caregivers and clinicians in the SONG-PD initiative21. However, despite PD-related infection being associated with increased morbidity and mortality21, a systematic review found that, out of 59 countries with dialysis registries, only 33 high- or middle-income countries monitored peritonitis rates26. Within these countries, global average peritonitis rates decreased from 0.6 episodes per patient-year (ppy) in 1992 to 0.3 episodes ppy in 2019; Asia–Pacific countries had the highest peritonitis rates followed by Europe, the Middle East and Africa. The lowest rate was reported in America (including North, South and Central America)26. Another systematic review (1980–2019) of data from seven African countries (17 studies, 1,894 participants) reported median peritonitis rates of 0.75 (95% CI 0.56–2.20) episodes ppy (specifically, 0.63 and 1.78 for adults and children, respectively)27. By contrast, a multicentre study that included 734 children in the USA, reported a peritonitis rate of 0.46 episodes ppy, with lower rates being associated with provider compliance with collaborative training and a quality assurance initiative that involved 29 paediatric centres in the US and was developed to facilitate uptake of standardized care bundles (that is, catheter insertion bundle, patient and caregiver training bundle, and catheter exit follow-up care bundle)28.
Differences in peritonitis rates across countries have been attributed to several modifiable factors, including practice patterns15. The Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS), which included 7,051 adult receiving PD patients from 209 centres across seven countries (Canada, Japan, Australia, New Zealand, Thailand, UK and the USA), reported appreciable differences in peritonitis rates (episodes ppy), for example, 0.26 in the USA, 0.27 in Japan, 0.35 in Australia and New Zealand, 0.38 in the UK and 0.40 in Thailand. Practices such as higher use of automated PD, prescription of antibiotics before catheter insertion, longer duration of PD training and larger facility size were associated with a lower risk of peritonitis15.
Gram-positive peritonitis predominates in most countries. In a 2011 registry analysis of 5,336 episodes of peritonitis in Australia and New Zealand, Gram-positive peritonitis accounted for 53.4% of episodes, with coagulase-negative staphylococci (27.2%) and Escherichia coli (6.3%) being the most common Gram-positive and negative organisms29, respectively. Similar findings were reported in 2006 for North America — 62.0% of infections in the USA and 61.0 % in Canada were caused by Gram-positive organisms (predominantly coagulase-negative staphylococci)30. By contrast, a 2014 study reported a 33.7% rate of Gram-positive peritonitis in Northern India31. An international 2020 study found lower proportions of Gram-positive peritonitis in Australia and New Zealand (39.0%), US (37.0%) and Canada (45.0%) than those reported in earlier studies. These subsequent rates were similar to those observed in Japan (37.0%), UK (38.0%) and Thailand (26.0 %)15; another 2020 study reported a 37.0% rate in Africa27, whereas a 2021 study from Spain32 reported infections in 55.9% of patients. Although the ISPD recommends that culture-negative peritonitis rates should be kept <15%23, most countries, including Canada (16.0%), Japan (21.0%), US (16.0 %)15 and India (18.2 %)28, were unable to achieve the target. Culture-negative peritonitis rates were also extremely high in Africa (28.7 %)27 and Thailand (28.0 %)15, and high antimicrobial resistance has been reported in parts of China33.
Peritonitis outcomes vary markedly across countries, including medical cure (69.0–80.7%), catheter removal (10.8–20.4%) and mortality (1.8–6.0 %)29,30,32,34. Of note, implementation of Continuous Quality Improvement (CQI) programmes can reduce peritonitis rates and improve outcomes35,36. Furthermore, peritonitis rates were relatively low when homemade PD solutions were used in an aseptic technique to treat patients with acute kidney injury (AKI), which has implications in low-resource settings37,38.
Exit-site or tunnel infections are important causes of peritonitis and subsequent technique failure39. In the PDOPPS, 8.5–20.8% of peritonitis episodes were associated with concomitant exit-site or tunnel infection15. These infections should therefore be monitored and reported as an important PD infection outcome in kidney registries and future studies.
Mortality
Overall, mortality on dialysis has gradually improved since the technique was introduced in the 1960s. Registry data suggest a contemporary adjusted 5-year survival of 52% and 42% for PD and HD, respectively40. Survival on PD has improved at a higher rate than that reported for HD. For example, from 2009–2019, the US Renal Data System (USRDS) reported an all-cause mortality decrease of 19.7% among all patients with kidney failure, including decreases of 10.5% for kidney transplant recipients, 17.5% for patients receiving HD and 21.3% for those receiving PD41. These improved survival rates for both dialysis modalities seem to be driven by a significant reduction in early mortality (within the first 2 years of dialysis initiation) owing to decreases in late dialysis referral and hospitalization rates. A 2017 review of the worldwide epidemiology of PD reported that 5-year patient survival varied between 48.4% and 64% across North America, Latin America, Europe and Oceania42.
The leading cause of mortality among patients receiving PD is CVD, which accounts for 52.7% of all deaths with a known cause41, followed by dialysis withdrawal (17.8%), sepsis (9.6%) and other causes (13.3%), including cancer, and gastrointestinal or respiratory disease41. Of note, the COVID-19 pandemic has partially counteracted decades of progress in mortality reduction in patients treated with kidney replacement therapy, owing to their high baseline prevalence of comorbidities and their vulnerability to severe COVID-19 (refs.43,44).
In terms of dialysis modality comparisons, some studies have suggested that the mortality risk was lower in patients receiving PD compared with HD, particularly in the first 2 years of dialysis45,46. These findings might be related to better preservation of RKF with PD than with HD (discussed below), but it might also reflect selection bias with residual confounding because some observational studies reported similar survival between PD and HD when only elective, outpatient, incident dialysis patients were analysed. Notably, patients who are frail or have a high comorbidity burden are more likely to initiate in-centre HD than a home therapy such as PD46. The time after dialysis initiation at which the relative survival benefit apparently switches from favouring PD to favouring HD is related to a number of factors including demographics (for example, age, sex and socioeconomic status), geography (for example, country and within-country centre variation), cause of kidney failure, dialysis vintage, comorbidity and centre effects or experience47. A large-scale study involving 398,940 patients who initiated dialysis in the USA (1995–2000) reported that the mortality risk was higher with HD than with PD in younger populations without substantial baseline comorbidity47. By contrast, HD was linked to a lower mortality rate than PD with increasing age and comorbidity index47. With PD, survival also seems to correlate positively with centre experience with PD48. Overall, the choice between PD and HD should be guided by the patients’ preferences, values and quality of life (QOL). Importantly, mortality comparisons between HD and PD require patient stratification according to major risk factors known to interact with treatment modality to avoid confounding effects. Moreover, the survival differences between HD and PD are not constant over time or across regions, but rather vary according to demographic, clinical, geographic, sociocultural and centre factors within and across regions.
In LICs, data on PD-related mortality are extremely limited13. In many of these countries, PD is either not used or used in <10% of patients needing KRT. Risk and mortality patterns also seem to differ from those of countries with higher incomes because in LICs, infection, rather than CVD, is reported to be the leading cause of mortality49.
Cardiovascular disease
The risk of CVD mortality among patients receiving dialysis is estimated to be 10- to 20-fold higher than that of the general population50. The types of CVD affecting these patients are diverse and range from atherosclerosis-related complications, such as acute coronary syndromes and stroke, to heart failure and arrhythmias.
The CVD risk factors in patients receiving PD can be broadly classified into three categories: general (‘traditional’), specific to patients with kidney failure (‘non-traditional’) and unique to PD. ‘Traditional’ CVD risk factors, including hypertension, dyslipidaemia and diabetes mellitus, are frequently present in patients with kidney disease. For example, in a cross-sectional study involving four PD centres in Greece, 95% of patients receiving PD had ambulatory hypertension and adequate blood pressure control was only achieved in 38.3% of patients51. Second, kidney dysfunction itself is a risk factor for CVD, which is evident even among patients with mild kidney dysfunction52, and has been attributed to ‘non-traditional’ CVD risk factors, such as inflammation, endothelial dysfunction and calcification53. For example, loss of kidney excretory function leads to the accumulation of advanced glycation end-products, which are thought to trigger the production of pro-inflammatory mediators, such as pro-atherogenic adhesion molecules that promote atherosclerosis, and thus contribute to the pathogenesis of vascular and kidney diseases54. Moreover, high levels of inflammatory markers (for example, CRP55, IL-6 (ref.56)) are associated with increased incidence of CVD. PD-specific risk factors include advanced glycation end-products present in PD solutions and volume overload caused by the loss of ultrafiltration57.
Despite the many CVD risk factors in patients receiving PD, no intervention has consistently or reliably reduced CVD burden in this population58. In 2020, 61.3% of people receiving PD in the USA were reported to have CVD and 52.7% of deaths in this group were attributed to CVD41,59. Specifically, the commonest cause of death among patients treated with PD in the USA was sudden cardiac death (39.7%)41; in patients treated with HD, sudden cardiac death accounted for 44.2% of deaths. Similarly, CVD was the most common cause of death among patients receiving PD in Australia (36%) and New Zealand (24%)60. However, the extent to which geographical variation influences the burden of CVD in PD remains uncertain owing to large disparities in measurement and reporting. Currently, less than half of the 79 registries that collect data from patients with kidney failure from 77 countries capture data related to CVD61. Moreover, disease definitions varied considerably across registries, which challenges direct comparison of CVD burden. For example, coronary artery disease (in the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA)), ischaemic heart disease (in the USRDS), myocardial infarction (in the Swiss Renal Registry) and angina pectoris (in the Finnish Registry for Kidney Diseases) have all been used to categorize the same disease entity61. The global community is awaiting the development and validation of a standardized core outcome measure for CVD in PD that can be implemented to better inform shared decision-making and improve outcomes.
Technique survival
PD technique survival refers to PD duration before transfer to HD, whereas technique failure refers to transfer to HD. Reported 3-year technique survival rates vary around the world from 29% to 91%62,63, but such variations have been attributed at least in part to differences in defining PD start date (for example, date of catheter insertion, training commencement or training completion), end date (for example, 1, 2 or 3 months after HD transfer) and inclusion of other relevant events (for example, death, transplantation or recovery of kidney function)25. A unified definition of technique survival has been proposed to include a composite end point of transfer to HD for >30 days or death64. Although technique survival is one of five core patient outcomes identified by the SONG-PD initiative21, a review of 120 PD RCTs showed that only 18% (n = 22) of studies reported technique survival17.
Factors associated with technique survival can be categorized as patient-related, centre-related and treatment-related25,65 (Box 2). Peritonitis and PD-related infections are the major cause of technique failure23,66,67 and accounted for 60% of HD transfers in a systematic review comprising 3,645 patients treated with PD25. Accordingly, a South African study that assessed PD outcomes from a predominantly rural area reported that patients with multiple episodes of peritonitis had a significantly higher likelihood of HD transfer than patients with 1 or no episodes (HR: 1.90; 95% CI: 1.04–3.47; P = 0.038)68. In other studies, reductions in peritonitis rates were accompanied by improvements in technique survival27,69. For instance, studies from seven African countries showed that, between the mid-1980s and late 2010s, peritonitis rates dropped from 2.72 to 0.44 episodes/patient year and 2-year technique survival improved from 50% to 90%27. Similar trends were reported by a French Language Peritoneal Dialysis Registry study of 14,673 patients who initiated PD — lower rates of peritonitis over time were accompanied by lower HD transfer rates69.
Centre effects are also a major factor in PD technique survival. In particular, larger PD centre size has been associated with better technique survival in numerous cohort studies in the Netherlands70, USA71, and Australia and New Zealand34. Having a higher proportion of patients treated with PD in a centre has also been associated with improved technique survival34,70. These findings might reflect greater cumulative PD experience, PD specialization or availability of a variety of clinical competence and experience (nurses, social workers, dietitians, surgeons and physicians). Other factors, such as adequate nutritional status72, use of automated PD73 and use of assisted PD74, have been associated with improved PD technique survival; this association was especially significant in paediatric age groups.
Technique failure is associated with a number of adverse outcomes. An ANZDATA analysis of all incident PD patients with technique failure between 1989 and 2014 showed overall mortality of 62%, with a significantly higher risk of death in those with technique failure related to infection or social reasons, compared with inadequate dialysis or mechanical issues (P < 0.0001)75. Moreover, an analysis of incident dialysis patients in Canada from 1999–2003 reported higher cost of care in those transferred from PD to HD than in those treated only with HD due to higher costs of dialysis provision, hospitalization, medications, and physician fees76.
There are no PD-specific strategies for improving technique survival. However, early interventions by dialysis centres to screen for modifiable risk factors for technique failure, such as diabetes mellitus and other comorbidities, extreme obesity, low adherence to PD prescriptions, low literacy, living in rural or remote areas, history of HD before PD, and previous episodes of peritonitis, have been suggested65.
Other infections
Compared with HD, PD is primarily home based and offers advantages in terms of reduced exposure to hospital-acquired infections. For example, various studies reported lower rates of SARS-CoV-2 infection in patients receiving PD than in those treated with HD77,78,79. In Wuhan, China, one study reported a SARS-CoV-2 infection incidence rate of 2.44 per 1,000 patient-months amongst patients receiving PD, which was similar to that of the general population77. Lower rates of SARS-CoV-2 infection in patients receiving PD than in those treated with HD were also reported in Italy (1.38% versus 3.55%, respectively)78 and in the UK (2.9% versus 9%)79.
PD is also associated with a lower risk of hepatitis B (HBV) and C (HCV) infections than HD80,81. In a study of 10 Asia–Pacific countries involving 201,590 patients (PD 27,802; HD 173,788), HCV prevalence ranged from 0.7 to 18.1% across countries, with lower HCV infection in PD populations than in those receiving HD (3.0% versus 7.9%), whereas HBV prevalence ranged from 1.35–14.6% with comparable prevalence between PD and HD80. A Brazilian study observed lower seroconversion rates with PD than with HD for HBV (0.01 versus 0.19 ppy) and HCV (0.03 versus 0.15 ppy)81. These differences probably reflected virus transmission within HD units.
Pneumonia is associated with high mortality among PD patients82. The incidence of pneumonia has been reported to be lower in PD than in HD in the USA (18.2 versus 29.0 ppy, respectively)83, but death due to pneumonia was similar in the two modalities in Australia and New Zealand (0.44 versus 0.43 per 100 patient-years)84.
Hospitalization
Hospitalization has been graded by patients, caregivers and clinicians in the SONG-PD initiative as a middle-tier outcome that is critically important to some stakeholder groups21. In the USA, hospitalization of patients receiving PD has fallen from 1.8 episodes ppy in 2009 to 1.5 episodes ppy in 2019, which appeared to have been partly driven by decreased peritonitis-related hospital admissions from 0.08 to 0.03 episodes ppy, respectively85. Importantly, between 2014 and 2017, most PD peritonitis episodes were managed by hospitalization in the USA (54.7%), Canada (51.7%), UK (64.7%), Japan (87.8%), Thailand (78.7%), and Australia and New Zealand (75.9 %)15. In Japan, peritonitis-related hospitalization (0.21 episodes ppy) was more common than cardiovascular-related hospitalization (0.16 episodes ppy)86, whereas the converse was true in the USA (0.03 episodes ppy versus 0.42 episodes ppy)85 and Canada (16% versus 28%)87. Hospitalization rates for infections other than peritonitis were 0.44 episodes ppy in 2019 in the USA85.
Encapsulating peritoneal sclerosis
EPS is a rare complication of PD that is characterized by intraperitoneal inflammation and fibrosis, and the development of a fibrocollagenous membrane that encases bowel loops, which leads to ultrafiltration failure and bowel obstruction, and increases mortality risk88. Reported incidence of EPS varies between 0.7 and 13.6 per 1,000 patient-years, depending on the population studied, owing to variation in demographic and clinical characteristics89,90. A substantial variation in EPS risk has also been reported both within and between countries owing to practice differences, including treatment protocols, fluid types and long-term dialysis duration; for example, the risk of EPS is generally low in the first 3–5 years after PD initiation88. Key risk factors for EPS include PD vintage, recurrent peritonitis, PD fluids (for example, the use of a dialysate with a high glucose concentration, an acetate buffer or a bioincompatible dialysis fluid) and medications (for example, the use of β-blockers or calcineurin inhibitors)91. Of note, the risk of EPS might be higher in kidney transplant recipients who used to receive PD than in patients who are still on PD and who have not received a transplant92. It is unclear whether this risk is related to the transplant procedure, reduced clearance of fibrin due to the cessation of peritoneal lavage following transplantation, or the known pro-fibrotic effects of calcineurin inhibitors92.
Mechanical complications
Approximately 40% of PD patients are estimated to develop mechanical complications93. Fluid leaks (including hydrothorax or pleuroperitoneal leaks) occur when PD solution leaks out of the peritoneal cavity94. These leaks happen with varying incidence according to differences in practice, population demographics and catheter types95,96. For example, the reported incidence of acute pleural effusion in patients receiving PD varies from 1.6 to 10%97 and women are affected more commonly than men98. Bowel obstruction is also a rare complication of PD that is most commonly seen in patients with EPS or previous history of abdominal surgery complicated by adhesions99. Catheter malpositioning could also affect optimal treatment delivery owing to inflow or outflow dysfunction. Notably, mechanical complications of PD are linked to patient and treatment outcomes as they can affect the timely initiation and sustainability of PD therapy.
Cognitive function
Cognitive dysfunction, including executive, memory, attention, information processing, language and visuospatial skill dysfunction, is common in patients receiving dialysis owing to multiple factors such as uraemia, electrolyte imbalance, comorbidity burden, homeostatic shifts with dialysis therapy, and vascular ischaemic changes that affect the brain100. The burden of cognitive dysfunction is estimated to be 3–5-fold higher in patients receiving dialysis than in the general population, and executive function is the domain that is most commonly affected101. Interestingly, PD is linked to better cognitive function than HD, particularly in the first few years of dialysis initiation, which has been attributed to it lower intensiveness, enhanced clearance of uraemic toxins and better anaemia control. Cognitive dysfunction is associated with increased risks of hospitalization, poor QOL, dialysis withdrawal and mortality101. In children, even subtle cognitive concerns can present barriers to learning, social functioning and overall QOL if not appropriately recognized or addressed.
Global patient-reported PD outcomes
Patient-reported outcomes give an indication of patient perceptions of how they function or feel, typically in relation to QOL or symptoms102. These outcomes are being increasingly incorporated into routine clinical care.
Life participation
Similar to other patients with kidney failure, patients receiving PD want to be able to live well, maintain their social roles and functioning, live as normal a life as possible, and maintain a sense of control over their health and wellbeing103. However, the daily and frequent nature of PD exchanges, as well as the increased risk of infections can limit the ability of these patients to participate in various life activities (for example, work, travel or recreation). Life participation, which is a SONG-PD core outcome21, is not uniformly assessed or reported across PD studies. A systematic review identified 42 different measures used for assessment of life participation, of which 36% were specifically designed to assess life participation and 64% assessed broader constructs, which suggests that these measures vary in their characteristics, content and validation24.
Obligatory dimensions refer to factors necessary for day-to-day living (for example, paid work, education, ability to perform household tasks), whereas non-obligatory dimensions refer to factors such as leisure activities. Although both dimensions are often reported together, the obligatory components are more likely to be reported than non-obligatory dimensions (for example, socializing and recreation)104,105,106,107. For instance, the SONG-PD group found that 76% of studies reported both dimensions; an additional 14% and 10% of studies reported obligatory and non-obligatory dimensions, respectively24. Patients receiving PD often have a better employment status than those treated with HD106,107,108,109,110, probably because they need to spend less time in a treatment facility. One study reported that PD was associated with a 4% increased probability of employment, 6% reduced probability of disability pension requirement and increased work income compared with HD111. However, studies from low-resource settings where maintenance PD is infrequently utilized showed much lower employment for PD patients compared with those receiving HD104. These regions might already be affected by low levels of employment in the general population and the reported unwillingness of employers to provide space for PD fluid storage or to provide time allowances for PD exchanges might further prevent patients receiving PD from access to employment104. There are no differences in non-obligatory dimensions, such as travel and recreation, between patients treated with HD and those treated with PD105. The ChinaQ study randomly assigned 725 patients across China to PD or HD and found that the burden of kidney disease on the PD group was non-inferior to that of the HD group112. However, studies comparing QOL between patients receiving HD or PD have tended to show better QOL in PD than in HD108,109,110. This difference might result from greater lifestyle flexibility, better ability to perform exchanges at home (or in a comfortable place that imposes less restriction), and better dietary flexibility in patients receiving PD110.
PD-related pain
Abdominal pain, which is a SONG-PD middle-tier outcome21, can occur during either the inflow or outflow phase of PD, particularly at PD initiation. Inflow pain often resolves with time on PD and is related to the acidic pH of conventional PD solutions and/or PD fluid turbulence during inflow113. In a systematic review, inflow pain was reduced by the use of PD solutions with neutral pH and low glucose degradation products (GDPs)114. Outflow pain near the end of the outflow phase, also known as drain pain, is related to suction on abdominal viscera or the peritoneum by the catheter tip. This type of pain is usually improved with tidal PD therapy, in which only part of the intraperitoneal fluid is exchanged to avoid the complete emptying of the peritoneal cavity. In a Canadian study that involved 375 patients receiving PD from six centres, 72 (19%) patients were administered tidal therapy, which specifically reduced drain pain115. Some studies have linked older age and abnormal bone mineral metabolism with PD-associated pain116.
Gastrointestinal symptoms
Most patients receiving PD experience gastrointestinal symptoms, including constipation (14.2–0.3%), indigestion (32.7%), early satiety (41.6%) and gastroesophageal reflux (30.7–93.1%)117. Compared with patients receiving HD, constipation is less common among patients treated with PD owing to higher dietary fibre and potassium intake, more liberal fluid consumption, more active lifestyle and lower use of phosphate binders and ion exchange resins118,119,120. Nonetheless, constipation is associated with higher risks of catheter malfunction, peritonitis and technique failure, which demands a proactive treatment approach118. Early satiety, postprandial pain and anorexia117,121,122 are more common in patients treated with PD owing to delayed gastric emptying123, which seems to be related to dialysate composition rather than intraperitoneal volume or pressure124. In particular, icodextrin and bicarbonate-based solutions reduce gastric hypomotility compared with glucose or lactate-based solutions123,124.
Fatigue
Fatigue affects between 42 and 89% of patients on dialysis125,126 and can lead to substantial social, mental and physical disability125,127 (Fig. 2). This outcome was identified as critically important in SONG-PD, mainly owing to its effect on life participation and carer burden21. Fatigue in patients treated with PD has been associated with older age128,129, female sex128,129, higher BMI127,129, unemployment status129, low physical activity130, anaemia and use of erythropoiesis-stimulating agents (ESAs)128,130,131, sleep disturbances126, poorer dialysis adequacy132, expression of serum markers of chronic inflammation and poor nutrition132, and early dropout133. An ongoing multicentre, adaptive RCT will test whether 12 weeks of structured exercise can reduce fatigue in 400 patients receiving PD or HD134.
The onset of fatigue in patients with kidney failure is multidimensional and multifactorial, with bidirectional and circular associations leading to substantial social, mental and physical disability. CRP, C-reactive protein; Hb, haemoglobin; KRT, kidney replacement therapy. Adapted with permission from Artom et al.125, Elsevier.
Depression
In an international cohort of 3,227 patients treated with PD, the prevalence of depression ranged from 28 to 40%; lowest in the USA, and highest in Japan and the UK135. Concerningly, an inverse relationship was observed between screen-positive and physician-diagnosed depression, highlighting problems of under-recognition and under-treatment135. Patients with kidney disease are affected by a multitude of complex inter-relational factors that can lead to the development of depression136,137 (Supplementary Figure 1). Lower functional status, younger age and cognitive impairment are especially common among PD patients with depression135. The evidence linking depression with mortality or HD transfer is inconclusive138,139,140 but several studies have highlighted its effect on QOL and peritonitis risk135,138.
Anxiety
Anxiety, which is defined as an emotional state in which a person experiences intense fear, uncertainty and apprehension towards a situation or event that is anticipated141, is reported in 24–43% of patients treated with PD139,142, particularly in men and patients with diabetes139, and is independently associated with death and HD transfer142. Fear of adverse events, social isolation, perceived financial stress from dialysis costs, caregiver burden and fear of HD transfer are important contributors to the development of chronic anxiety in these patients143. Ensuring appropriate patient selection for PD, providing comprehensive and early pre-dialysis education, and supporting patients with a multidisciplinary network are crucial measures for minimizing anxiety in this population143.
Cramps
Cramps are characterized by sudden, involuntary, painful and prolonged muscular contractions117,121,122. Although cramps are probably under-recognized in PD, a 2012 study revealed that 73% of patients on PD for ≥3 months experienced cramping, which is comparable with what is observed in HD144. Both modalities share common factors implicated in dialysis-associated cramps, including plasma volume contraction, metabolic alkalosis, hypotension, hyponatraemia, carnitine deficiency and hypomagnesaemia145,146.
Pruritus
More than half of all patients receiving PD (52.1–62.6%) experience pruritus147,148, which is characterized by an itching sensation with variable spatial distribution (usually affecting large, discontinuous areas of skin) and without evident skin alterations that is exacerbated at night149. Pruritus is associated with impaired QOL domains, particularly sleep, mood and social functioning150, and increased risks of death and HD transfer151. Although the pathogenesis of pruritus remains largely unknown, it is no longer thought to be purely histamine-mediated but rather to result from a complex crosstalk between dermal mast cells, epidermal keratinocytes, T helper 1 lymphocytes and nerve fibres152.
Restless legs syndrome
Restless legs syndrome (RLS) is a clinical diagnosis based on an urge to move the legs, often accompanied by an uncomfortable sensation at rest, that improves with activity and worsens in the evening or at night153. Using a broad definition, RLS was identified in >50% of patients receiving dialysis154, whereas using the stricter International Restless Legs Syndrome Study Group (IRLSSG) diagnostic criteria yielded a prevalence of 10–20%154. RLS prevalence did not differ between PD and HD155.
Sexual function
Sexual dysfunction is common in chronic kidney disease (CKD) and correlates inversely with estimated glomerular filtration rate156. In a systematic review of 50 observational studies of sexual dysfunction in CKD populations, 16 studies (429 patients) included PD patients157. Most studies focused solely on men and specifically on erectile dysfunction157. The pooled analysis showed that the prevalence of erectile dysfunction in patients treated with PD was 64%157, compared with 79% in patients receiving HD and 59% in kidney transplant recipients (heterogeneity P = 0.2)157.
Sleep quality
Sleep disorders are common in patients receiving dialysis, can affect QOL substantially, and are associated with fatigue and depression158. Several studies have reported sleep quality outcomes in patients treated with PD, but they used variable measurement methods. The Pittsburgh Sleep Quality Index (PSQI), which is a standardized self-administered sleep questionnaire, was used in 6 studies159,160,161,162,163,164,165 (Supplementary Table 1). The mean prevalences of poor sleep quality, defined by PSQI >5 (n = 3) and PSQI ≥5 (n = 3), were 69.37%% and 81%, respectively (Supplementary Table 1). Whether sleep quality differs between patients undergoing PD and those on HD remains uncertain, although two small studies165 (n = 102 and 124) did not report appreciable differences159,162.
Global surrogate PD outcomes
Surrogate outcomes are biological or physiological parameters used in the prediction of risk of hard adverse clinical events, such as kidney failure, CVD and mortality, or clinical benefits, such as reduction in the risk of adverse health outcomes, based on epidemiological (for example, co-morbidities such as anaemia), therapeutic (for example, blood pressure treatment) or pathophysiological evidence (for example, inflammatory markers such as serum C-reactive protein) that may or may not be validated166. In this section, we review key surrogate outcomes of relevance in predicting risk of adverse health outcomes in patients on PD. Hard endpoints such as CVD events or mortality take longer to reach, and thus surrogate measures are important intermediate measures of risk of prognostic and therapeutic significance.
Residual kidney function
Residual kidney function (RKF) is vital for patients treated with PD because it is strongly associated with improved survival and technique survival167. In general, PD is thought to preserve RKF better than HD because it does not commonly induce intradialytic hypotension and/or hypovolaemia168. Other PD-specific interventions associated with better RKF preservation include the use of solutions with neutral pH and low GDPs169, and incremental PD170; these interventions seem to be beneficial owing to reduced exposure to GDP and glucose, which can be nephrotoxic with attendant fibrotic changes and loss of kidney function171. The status of RKF at PD initiation varies across countries; in PDOPPS, the median 24-h urine volume ranged from 0.4 l (Thailand; interquartile range 0.08–0.8) to 1.2 l (UK; IQR 0.71–1.77)15. The rate of RKF decline reportedly decreases after PD initiation (−2.69 ± 0.18 ml/min/1.73 m2/year) compared with the pre-dialysis rate (−4.09 ± 0.33 ml/min/1.73 m2/year, P < 0.001)172.
Fluid volume status
Volume overload in PD is associated with accelerated RKF decline, HD transfer and mortality173,174,175. The use of hypertonic solutions and automated PD was not associated with significant volume reductions in the Patient Outcomes in Dialysis-Peritoneal Dialysis study, which involved 1,054 incident patients from 135 centres in 28 countries (3-year follow-up)174. Based on the findings of a 2018 Cochrane systematic review114, the ISPD Guidelines make a strong level 1 A recommendation that use of neutral pH, low GDP (‘biocompatible’) PD solutions improves preservation of RKF and urine output176. These guidelines also strongly recommend (level B1) that icodextrin should be considered as an alternative to hypertonic solutions to maintain euvolaemia in patients with inadequate ultrafiltration. Compared with clinical assessment alone, the use of bioimpedance devices does not improve guidance of fluid management and PD prescription significantly173,177.
Blood pressure
The prevalence of hypertension in patients treated with PD varies from 29 to 88% in different studies178,179. When PD is started, blood pressure control is frequently better than in patients starting HD owing to better volume control homeostasis and reduced haemodynamic shifts compared with HD, although this difference is often not maintained over time180. Similar to several HD studies181, a USRDS study found a non-linear relationship between blood pressure and survival in patients treated with PD182. Specifically, a systolic pressure <111 mmHg was associated with increased mortality, whereas hospitalization duration was shorter in patients with a systolic pressure >120 mmHg182. However, this protective effect was not observed in another study183. These discrepant findings might be explained by the heterogeneous nature of the population across the two studies. Although no high-certainty evidence exists, guidelines recommend that patients receiving PD with blood pressure >140/90 mmHg should be treated to maintain their BP <140/90 mmHg58. Of note, hypertension in patients treated with PD is mechanistically linked to salt and water retention184; therefore, volume status should be optimized before starting or increasing anti-hypertensive medications58,185.
CKD mineral and bone disorder
CKD mineral and bone disorder refers to any one or a combination of abnormalities of mineral metabolism (calcium, phosphorus, vitamin D, parathyroid hormone (PTH)), bone metabolism (kidney osteodystrophy, alkaline phosphatase) and/or vascular calcification186. These complications increase the risk of fractures, CVD and mortality in patients receiving dialysis187,188,189. Despite physiological differences in mineral metabolism between patients treated with PD and those treated with HD190, most studies have focused on HD populations, with relatively few studies including PD patients191,192,193.
Anaemia
Anaemia is a common multifactorial complication in patients with kidney failure driven, for example, by low erythropoietin production, iron deficiency and inflammation. Untreated or inadequately treated anaemia can lead to reduced QOL and increased risk of CVD and health care utilization194. Patients treated with PD often need to use fewer ESAs to treat anaemia than patients receiving HD (71.4% versus 96.9%, respectively, n = 274,784; US data)195. These differences have been attributed to better RKF preservation in patients receiving PD, who are also at a lower risk of blood loss than patients receiving HD. The prevalence of ESA-requiring anaemia varies geographically (for example, 82% in Hong Kong versus 96% in Thailand)196. However, the true extent of variation is incompletely understood, especially in LICs and LMICs, where haemoglobin is often never measured (LICs, 1/5 countries (20%); LMICs, 4/17 (24%))4. Of note, hypoxia-inducible factor inhibitors (for example, roxadustat) can improve anaemia and other clinical parameters, including decline of RKF, in patients receiving PD197.
Other outcomes
Additional socio-economic (non-clinical) factors that affect the day-to-day lives of people receiving PD, as well as their families, caregivers and friends, should also be considered.
Effects on family and friends
The regular dialysis sessions and responsibilities associated with PD can be overwhelming and can extend naturally to family, friends and caregivers of patients198,199. Thus, minimizing patient and caregiver fatigue to improve QOL, increase patients’ adoption of PD and decrease HD transfer is important200. In a PDOPPS cohort (n = 2,760), “space taken up by PD supplies” was the most commonly cited disadvantage of PD and had the strongest association with HD transfer (hazard ratio 1.28; 95% confidence interval 1.07–1.53)201. Developing approaches that enable the reduction of total PD fluid storage requirements would be prudent to overcome this barrier to PD adoption and improve PD patient experience201. Moreover, advances in technology could decrease the burden of PD care at home. In one study, patients and their care partners favoured remote PD management to troubleshoot problems and decrease clinic visits202.
Finances
The cost of providing KRT remains an important barrier to care access for patients with kidney failure in many parts of the world203,204. According to data available from 87 countries, the average annual cost of maintenance PD was 20,524 international dollars (equivalent to the value of US dollars in 2016) per ppy205. However, costs varied widely within countries and by World Bank income group. Using the same data, the cost ratio of HD to PD was >1 (that is, PD was less expensive) in 59% of countries205. More than half of HICs (65%) and upper-middle-income countries (62%) had a cost ratio >1 but 39% of LMICs had similar HD to PD costs (that is, ratios ~1)205. No data were available for LICs. Other studies also found that PD was less costly than HD206,207. Low-cost PD equipment (either through low-cost manufacturing or import) is needed to increase dialysis uptake in LICs5,204.
PD outcomes in vulnerable populations
Certain population subgroups might be particularly disadvantaged because of their biological characteristics and social circumstances, including literacy, economic status, living conditions and access to health insurance and health care. This section focuses on the variability and impact of PD outcomes in vulnerable population subgroups, such as children and adolescents, women and Indigenous peoples.
Children
PD is often selected as the initial KRT modality in the paediatric population, usually as a bridge to transplantation208. Urgent start of PD for AKI is also often used in children, especially in low-resource regions, as this may be the only form of dialysis available209. PD was leveraged as a KRT modality for paediatric AKI, more commonly in LMICs than in HICs. Acute PD for AKI is often started using improvised catheters and homemade fluid210. In the majority of cases, patients recover from AKI but when they do not, chronic PD must be introduced. Survival on PD is higher in children than in adults. A USRDS analysis reported 5-year survival of 76% and 85% in patients treated with PD aged 0–9 and 10–14 years, respectively208. Survival amongst infants on PD is 4-fold lower than among older children211. According to the North American Pediatric Renal Trials and Collaborative Studies data, patients who started PD in infancy had a 3-year survival probability of 74.6%, compared with 96.2% for those who started at an age >12 years212. Notably, infant survival was significantly better from 2000 to 2012 than from 1990 to 1999 (ref.213). In Italy, younger children (0–5 years) also had poorer survival and technique survival than older children (5–15 years)214.
Apart from age, survival on PD also varies geographically. In the International Pediatric Peritoneal Dialysis Network Registry (2,956 children, mean age 7.6 years), the 3-year probability of death varied between 2% (North America) and 9% (Eastern Europe)215. Mortality was higher in LICs, and about half of the variance was explained by country income category215. In a report from India, the 3-year survival amongst 66 children started on PD was only 30%, with peritonitis being the main cause of death216. Unlike in HICs, where children are usually treated with automated PD overnight to facilitate school attendance and play during the day, most children in LICs are treated with manual exchanges.
Growth is another important concern in children on PD and is affected by nutrition and RKF; the use of recombinant growth hormone might be required to ensure adequate growth217. Higher fill volumes, fewer peritonitis episodes and the use of biocompatible fluids were associated with improved growth in small studies211.
Women
Men outnumber women by 2:1 in the dialysis population218. However, data on sex differences in the characteristics, treatment and outcomes of PD are scarce. According to data from the Andalusian SICATA Registry, women on PD had similar overall mortality (adjusted HR 0.91, 95% CI 0.72–1.15), higher infection-specific mortality (adjusted HR 1.76, 95% CI 1.03–3.01) and similar cardiovascular mortality (adjusted HR 0.76, 95% CI 0.52–1.09) compared with men219. In another study from Australia involving 506 patients, female sex doubled the peritonitis risk (OR 1.91 95% CI 1.2–3.01)220.
Data on the impact of sex on technique failure are inconsistent. A lower risk of technique failure was reported in females receiving PD in Germany (HR 0.66, CI 0.506–0.89, P = 0.005)221, the USA (HR 0.78, 95% CI 0.64–0.95)222, and Australia and New Zealand (HR for males 1.13, CI 1.06–1.20)223; however, other studies failed to find any sex differences in technique failure224.
Pregnancy, although uncommon, presents a unique challenge for women on PD. In a review of 222 pregnancies in 208 women receiving dialysis (14 on PD)225, the rate of successful live births was similar in women on HD to those on PD (79%). Four of the 14 patients treated with PD developed peritonitis. Of note, bloody effluent is a harbinger of serious complications — 2 out of 3 pregnancies complicated by haemoperitoneum eventually resulted in miscarriage.
Indigenous peoples
The burden of kidney failure is greatly increased in Indigenous peoples, most of whom live in remote locations. Although PD offers several advantages in this context, such as proximity to family and community support, reduced travel times and elimination of the need to relocate, the use and outcomes of PD in Indigenous populations are highly variable and often poor. Compared with non-Indigenous populations, the proportion of patients starting KRT with PD in 2009 was considerably lower in Australian Aboriginal and Torres Strait Islander peoples (18% versus 25%) and New Zealand Māori (31% versus 41%)226. In Canada, Indigenous people with kidney disease were half as likely to be on PD as white patients (OR 0.51, 95% CI 0.40–0.65)227. A number of factors, including low socioeconomic status, literacy, colonialism and geography (for example, living in remote or rural communities), are associated with lower rates of PD use in Indigenous populations compared with non-Indigenous populations.
Clinical outcomes are also generally poorer in Indigenous patients228. Data from Australia and New Zealand revealed higher mortality amongst Indigenous than amongst non-Indigenous patients receiving PD (HR 1.23, 95% CI 1.01–1.50; P < 0.05), after adjustment for patient demographics, comorbidities and peritoneal solute transport characteristics229. Indigenous patients also had higher peritonitis rates (1.14 versus 0.71 episodes per year, P < 0.001), shorter time to first peritonitis episode (9.9 versus 19.3 months; P < 0.001) and a higher level of technique failure (HR 1.30, 95% CI 1.15–1.47; P < 0.001) than non-Indigenous patients230. Similarly, Canadian Aboriginal patients had a non-significantly increased risk of technique failure (HR, 1.46; 95% CI, 0.95 to 2.23; P = 0.08) and comparable overall mortality227. Some evidence suggests that PD outcomes in Indigenous people are influenced by their location, with higher rates of peritonitis, technique failure and mortality reportedly associated with remote residence231. Importantly, 79% of Indigenous Australian patients who started PD between 1995 and 2008 lived remotely231. The relatively poor outcomes in these populations have been attributed to non-medical factors, such as poverty, unemployment, crowded living situations and lack of availability of speciality care.
Older patients
As a result of global increases in life expectancies, the number of older people (≥65 years old) commencing dialysis is growing232. Despite the potential advantages of PD listed in the introduction of this review, older patients face several potential barriers to accessing PD, including frequent late presentation, comorbidities, frailty, functional dependence, impaired dexterity, impaired visual acuity and reduced cognitive function233,234. A systematic review and meta-analysis of 14 non-randomized studies from 13 countries in Europe, Asia, Latin America and Oceania between 2000 and 2021 reported that (with low-certainty evidence) PD in older patients might be associated with higher mortality (relative risk (RR) 2.45, 95% CI 1.36–4.40, P = 0.003, I2 = 97%) and more frequent peritonitis (RR 1.56, 95% CI 1.18–2.07, P = 0.002, I2 = 76%); differences in technique survival between older and younger patients were minimal or absent (RR 0.95, 95% CI 0.86–1.05, P = 0.32, I2 = 86%)233.
The use of assisted PD, whereby a carer performs PD for the patient, could potentially circumvent barriers to PD in older patients. However, in a systematic review of 34 non-randomized studies involving 46,597 participants from 20 jurisdictions, the relative efficacy and safety of assisted PD were uncertain, owing to highly variable study quality and markedly heterogeneous reported outcomes235. Similarly, a 2021 narrative review concluded that a difference in QOL, mortality or hospitalization between patients on assisted PD and those on facility HD was uncertain, after adjusting for the fact that patients receiving assisted PD were older and more frail236. Of note, assisted PD was significantly cheaper than facility HD in Canada and Western Europe236.
Centre effects and PD outcomes
Despite improvements in PD care over the years, consistent and unacceptable variation in outcomes between different PD centres within various countries remains29,237. Although these differences have been previously attributed to heterogeneity in patient-related factors, emerging evidence suggests that variations in PD centre characteristics have a much greater role.
An ANZDATA registry study that included 54,773 patients with kidney failure reported 0–87% variation in the uptake of home dialysis (n = 24,399; 88.4% PD) across 76 centres238. Centre-level predictors of low uptake included small centre size, a small proportion of patients with permanent HD access at dialysis initiation and low weekly facility HD hours, defined in the study as ≤12.6 h of time spent on HD. The variation in odds of home dialysis uptake in this Australian study across centres was associated with centre-level characteristics (24%) and not patient-level characteristics238. This dominant effect of centre-level characteristics was observed for several PD outcomes, including peritonitis occurrence239, peritonitis outcomes34 and technique survival240. Other studies from France have reported similar results that support the important effects of centre-level characteristics on PD outcomes237. Centre-level characteristics consistently associated with better PD outcomes include a high proportion of PD patients in the centre, which is a marker of greater clinical experience with PD34,240 and alignment of centre practices with ISPD guideline recommendations (for example, the use of empiric antibiotic therapy against both Gram-positive and Gram-negative organisms in patients with peritonitis)34. These results highlight the importance of implementing interventions at a centre level to ensure that practices follow standardized, evidence-informed policy.
Prescribing high-quality goal-directed PD
In 2020, the ISPD published practice recommendations for prescribing high-quality goal-directed PD176. These recommendations represented a paradigm shift from the conventional, non-evidence-based and potentially harmful practice of prescribing PD to achieve so-called ‘dialysis adequacy’ based primarily on the unvalidated and imprecise surrogate measure of small solute clearance. This approach potentially led to greater PD-related burden and complications without clear evidence supporting its benefit. Instead, the new guidelines advocate a tailored, shared decision-making model. Such a PD plan should be developed by the person receiving PD in collaboration with their care team to ensure the delivery of high-quality PD that helps the patient to achieve their expressed, personal goals of care, and is informed by careful assessment of (in descending order of priority) patient-reported outcome measures (such as QOL and symptom burden), clinical measures (such as fluid status) and, to a much lesser extent, surrogate measures (such as RKF, bone mineral disorder parameters, nutritional indices, peritoneal membrane function and small solute clearance) (Supplementary Figure 2). Of note, the guidelines indicate that small solute clearance measurement should not, in and of itself, influence PD prescription. The ISPD guidelines advocate for a quality cycle in which there is iterative evaluation of whether or not a patient’s goals of care are being met, taking into consideration a number of hierarchical PD outcome measures (Supplementary Figure 2).
Conclusions
PD remains an important treatment modality but its adoption as a treatment modality for kidney failure varies widely across the world. The median global prevalence of PD has been estimated at 38.1 pmp but varied over 5,000-fold from as low as 0.1 pmp in Egypt to 531 pmp in Hong Kong. Interestingly, the majority of patients on PD resided in only four countries — China, USA, Mexico and Thailand.
The association of PD with better clinical and patient-reported outcomes compared with HD is well established. These benefits include better preservation of RKF, enhanced patient satisfaction, improved QOL, better kidney transplantation outcomes (in transplant recipients), a delayed need for vascular access (especially in small children), enhanced anaemia management and lower risk of blood-borne and respiratory virus infections, including the novel SARS-CoV-2. Of note, the nephrology community has worked assiduously to improve dialysis outcomes, such that over the last decade PD outcomes have been improving in many parts of the globe, However, significant variability in the epidemiology of these outcomes still exists across regions and countries, largely driven by patient-, centre- and system-level inequities, and differences in practice culture and resource allocation to PD. Enactment of strategies for improvement and monitoring of outcomes via enhanced standardization, monitoring and reporting, as well as implementation of CQI initiatives and novel interventions, including incremental PD, the use of biocompatible PD solutions and remote PD monitoring, are all crucial to improving PD outcomes.
References
Pecoits-Filho, R. et al. Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney Int. Suppl. 10, e3–e9 (2020).
Bello, A. K. et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 367, l5873 (2019).
Bello, A. K. et al. Assessment of global kidney health care status. JAMA 317, 1864–1881 (2017).
Cho, Y. et al. Peritoneal dialysis use and practice patterns: an international survey study. Am. J. Kidney Dis. 77, 315–325 (2021).
Karopadi, A. N., Mason, G., Rettore, E. & Ronco, C. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol. Dial. Transpl. 28, 2553–2569 (2013).
Johnson, D. W. et al. Renal services disaster planning: lessons learnt from the 2011 Queensland floods and North Queensland cyclone experiences. Nephrology 18, 41–46 (2013).
Chang, Y. T. et al. Cost-effectiveness of hemodialysis and peritoneal dialysis: a national cohort study with 14 years follow-up and matched for comorbidities and propensity score. Sci. Rep. 6, 30266 (2016).
Juergensen, E. et al. Hemodialysis and peritoneal dialysis: patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin. J. Am. Soc. Nephrol. 1, 1191–1196 (2006).
Li, P. K. & Chow, K. M. Peritoneal dialysis-first policy made successful: perspectives and actions. Am. J. kidney Dis. 62, 993–1005 (2013).
Liu, F. X. et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit. Dial. Int. 35, 406–420 (2015).
Sedor, J. R. et al. ASN End-Stage Renal Disease Task Force: perspective on prospective payments for renal dialysis facilities. J. Am. Soc. Nephrol. 21, 1235–1237 (2010).
Tantivess, S., Werayingyong, P., Chuengsaman, P. & Teerawattananon, Y. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ 346, f462 (2013).
Okpechi, I. G. et al. The case for increased peritoneal dialysis utilization in low- and lower-middle-income countries. Nephrology (2022).
Al Sahlawi, M. et al. Variation in peritoneal dialysis-related peritonitis outcomes in the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am. J. Kidney Dis. 79, 45–55.e41 (2022).
Perl, J. et al. Peritoneal dialysis-related infection rates and outcomes: results from the Peritoneal Dialysis Outcomes and Practice Patterns Study (PDOPPS). Am. J. Kidney Dis. (2020).
Cho, Y. & Johnson, D. W. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am. J. Kidney Dis. 64, 278–289 (2014).
Manera, K. E. et al. Scope and heterogeneity of outcomes reported in randomized trials in patients receiving peritoneal dialysis. Clin. Kidney J. 14, 1817–1825 (2021).
Manera, K. E. et al. Standardized Outcomes in Nephrology-Peritoneal Dialysis (SONG-PD): Study Protocol for Establishing a Core Outcome Set in PD. Perit. Dial. Int. 37, 639–647 (2017).
Manera, K. E. et al. Patient and Caregiver Priorities for Outcomes in Peritoneal Dialysis: multinational nominal group technique study. Clin. J. Am. Soc. Nephrol. 14, 74–83 (2019).
Manera, K. E. et al. An international Delphi survey helped develop consensus-based core outcome domains for trials in peritoneal dialysis. Kidney Int. 96, 699–710 (2019).
Manera, K. E. et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology–Peritoneal Dialysis) Consensus Workshop. Am. J. Kidney Dis. 75, 404–412 (2020).
Tong, A. et al. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop. Am. J. Kidney Dis. 69, 97–107 (2017).
Li, P. K. et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit. Dial. Int. 42, 110–153 (2022).
Manera, K. E. et al. Patient-reported outcome measures for life participation in peritoneal dialysis: a systematic review. Nephrol. Dial. Transpl. 36, 890–901 (2021).
Elphick, E. et al. Outcome measures for technique survival reported in peritoneal dialysis: a systematic review. Perit. Dial. Int. 42, 279–287 (2021).
Marshall, M. R. A systematic review of peritoneal dialysis-related peritonitis rates over time from national or regional population-based registries and databases. Perit. Dial. Int. 42, 39–47 (2022).
Okpechi, I. G. et al. Prevalence of peritonitis and mortality in patients with ESKD treated with chronic peritoneal dialysis in Africa: a systematic review. BMJ Open. 10, e039970 (2020).
Sethna, C. B. et al. Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE Collaborative. Clin. J. Am. Soc. Nephrol. 11, 1590–1596 (2016).
Ghali, J. R. et al. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit. Dial. Int. 31, 651–662 (2011).
Mujais, S. Microbiology and outcomes of peritonitis in North America. Kidney Int. Suppl., S55–S62, (2006).
Prasad, K. N. et al. Microbiology and outcomes of peritonitis in northern India. Perit. Dial. Int. 34, 188–194 (2014).
de la Espada Piña, V. et al. Two decades of analysis of peritonitis in peritoneal dialysis in Andalusia: Epidemiological, clinical, microbiological and progression aspects. Nefrología 41, 417–425 (2021).
Wu, H. et al. Changes of antibiotic resistance over time among Escherichia coli peritonitis in Southern China. Perit. Dial. Int. 42, 218–222 (2022).
Htay, H. et al. Center effects and peritoneal dialysis peritonitis outcomes: analysis of a national registry. Am. J. Kidney Dis. 71, 814–821 (2018).
Wang, J. et al. Implementation of a continuous quality improvement program reduces the occurrence of peritonitis in PD. Ren. Fail. 36, 1029–1032 (2014).
Yu, Y. et al. Impact of continuous quality improvement initiatives on clinical outcomes in peritoneal dialysis. Perit. Dial. Int. 34 (Suppl 2), S43–S48 (2014).
McCulloch, M. I., Nourse, P. & Argent, A. C. Use of locally prepared peritoneal dialysis (PD) fluid for acute PD in children and infants in Africa. Perit. Dialysis Int. 40, 441–445 (2020).
Nkoy, A. B. et al. A promising pediatric peritoneal dialysis experience in a resource-limited setting with the support of saving young lives program. Perit. Dial. Int. 40, 504–508 (2020).
Lin, J. et al. Prevalence and risk factors of exit-site infection in incident peritoneal dialysis patients. Perit. Dial. Int. 40, 164–170 (2020).
United States Renal Data System. 2018 Annual Report: End Stage Renal Disease Ch. 5: Mortality, https://usrds.org/media/1730/v2_c05_mortality_18_usrds.pdf (2018).
United States Renal Data System. 2020 Annual Report: End Stage Renal Disease Ch. 5: Mortality, https://adr.usrds.org/2020/end-stage-renal-disease/5-mortality (2021).
Li, P. K. et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13, 90–103 (2017).
Taji, L. et al. COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ 193, E278–e284 (2021).
Weinhandl, E. D. et al. Initial effects of COVID-19 on patients with ESKD. J. Am. Soc. Nephrol. 32, 1444–1453 (2021).
Termorshuizen, F. et al. Hemodialysis and peritoneal dialysis: comparison of adjusted mortality rates according to the duration of dialysis: analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis 2. J. Am. Soc. Nephrol. 14, 2851–2860 (2003).
Noordzij, M. & Jager, K. J. Survival comparisons between haemodialysis and peritoneal dialysis. Nephrol. Dial. Transpl. 27, 3385–3387 (2012).
Vonesh, E. F., Snyder, J. J., Foley, R. N. & Collins, A. J. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 66, 2389–2401 (2004).
Schaubel, D. E., Blake, P. G. & Fenton, S. S. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int. 60, 1517–1524 (2001).
Tamayo Isla, R. A. et al. Baseline predictors of mortality among predominantly rural-dwelling end-stage renal disease patients on chronic dialysis therapies in Limpopo, South Africa. PLoS One 11, e0156642 (2016).
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32, S112–S119 (1998).
Vareta, G. et al. Epidemiology of hypertension among patients on peritoneal dialysis using standardized office and ambulatory blood pressure recordings. Am. J. Nephrol. 53, 139–147 (2022).
Henry, R. M. et al. Mild renal insufficiency is associated with increased cardiovascular mortality: the Hoorn Study. Kidney Int. 62, 1402–1407 (2002).
Kendrick, J. & Chonchol, M. B. Nontraditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 4, 672–681 (2008).
Hegab, Z., Gibbons, S., Neyses, L. & Mamas, M. A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 4, 90–102 (2012).
Herzig, K. A. et al. Is C-reactive protein a useful predictor of outcome in peritoneal dialysis patients. J. Am. Soc. Nephrol. 12, 814–821 (2001).
Cho, Y. et al. Baseline serum interleukin-6 predicts cardiovascular events in incident peritoneal dialysis patients. Perit. Dial. Int. 35, 35–42 (2015).
Hung, S. C. et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 85, 703–709 (2014).
Wang, A. Y. et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients part I — assessment and management of various cardiovascular risk factors. Perit. Dial. Int. 35, 379–387 (2015).
United States Renal Data System. 2020 Annual Report: End Stage Renal Disease Ch. 8: Cardiovascular disease in patients with ESRD, https://adr.usrds.org/2020/end-stage-renal-disease/8-cardiovascular-disease-in-patients-with-esrd (2021).
ANZDATA Registry. 44th Report Ch. 3: Mortality in kidney failure with replacement therapy (Australia and New Zealand Dialysis and Transplant Registry, 2021).
Ng, M. S. Y., Charu, V., Johnson, D. W., O’Shaughnessy, M. M. & Mallett, A. J. National and international kidney failure registries: characteristics, commonalities, and contrasts. Kidney Int. 101, 23–35 (2022).
Jose, M. D. et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology 16, 19–29 (2011).
Wu, C. et al. Peritoneal dialysis in Sichuan province of China — report from the Chinese National Renal Data System. Ren. Fail. 40, 577–582 (2018).
Lan, P. G. et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit. Dial. Int. 36, 623–630 (2016).
Da Luz, L. G., Ankawi, G., Digvijay, K., Rosner, M. H. & Ronco, C. Technique failure in peritoneal dialysis: etiologies and risk assessment. Blood Purif. 50, 42–49 (2021).
Li, P. K. et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit. Dial. Int. 36, 481–508 (2016).
Lan, P. G., Clayton, P. A., Saunders, J., Polkinghorne, K. R. & Snelling, P. L. Predictors and outcomes of transfers from peritoneal dialysis to hemodialysis. Perit. Dial. Int. 35, 306–315 (2015).
Isla, R. A. T. et al. Continuous ambulatory peritoneal dialysis in Limpopo Province, South Africa: predictors of patient and technique survival. Perit. Dial. Int. 34, 518–525 (2014).
Boyer, A. et al. Trends in peritoneal dialysis technique survival, death, and transfer to hemodialysis: a decade of data from the RDPLF. Am. J. Nephrol. 52, 318–327 (2021).
Huisman, R. M., Nieuwenhuizen, M. G. & Th de Charro, F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol. Dial. Transpl. 17, 1655–1660 (2002).
Afolalu, B. et al. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit. Dial. Int. 29, 292–296 (2009).
Yang, Y. et al. Predictive value of objective nutritional indexes in technique failure in peritoneal dialysis patients. J. Ren. Nutr. https://doi.org/10.1053/j.jrn.2021.09.005 (2021).
Wang, I. K. et al. Comparison of patient survival and technique survival between continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Perit. Dial. Int. 40, 563–572 (2020).
Song, Q. et al. Assisted peritoneal dialysis: a feasible KRT modality for frail older patients with end-stage kidney disease (ESKD). Sci. Rep. 11, 14928 (2021).
Chen, J. H. C., Johnson, D. W., Hawley, C., Boudville, N. & Lim, W. H. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci. Rep. 8, 3980 (2018).
Chui, B. K. et al. Health care costs of peritoneal dialysis technique failure and dialysis modality switching. Am. J. Kidney Dis. 61, 104–111 (2013).
Jiang, H. J. et al. COVID-19 in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 16, 121–123 (2020).
Quintaliani, G. et al. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society of Nephrology. J. Nephrol. 33, 725–736 (2020).
UK Renal Registry. COVID-19 surveillance report for renal centres in the UK: All regions and centres. https://renal.org/sites/renal.org/files/ALL_REGIONS_CENTRES_covid_report_29122020.pdf (2020).
Johnson, D. W. et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol. Dial. Transpl. 24, 1598–1603 (2009).
Cendoroglo Neto, M. et al. Incidence of and risk factors for hepatitis B virus and hepatitis C virus infection among haemodialysis and CAPD patients: evidence for environmental transmission. Nephrol. Dial. Transpl. 10, 240–246 (1995).
He, F. et al. Pneumonia and mortality risk in continuous ambulatory peritoneal dialysis patients with diabetic nephropathy. PLoS One 8, e61497 (2013).
Guo, H., Liu, J., Collins, A. J. & Foley, R. N. Pneumonia in incident dialysis patients — the United States Renal Data System. Nephrol. Dial. Transpl. 23, 680–686 (2008).
Johnson, D. W. et al. Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am. J. Kidney Dis. 53, 290–297 (2009).
United States Renal Data System. Annual Data Report: End Stage Renal Disease Ch. 5: Hospitalization, https://adr.usrds.org/2021/end-stage-renal-disease/5-hospitalization (2021).
Kawanishi, H. et al. Mortality, hospitalization and transfer to haemodialysis and hybrid therapy, in Japanese peritoneal dialysis patients. Perit. Dial. Int. 42, 305–313 (2021).
Laurin, L. P. et al. Outcomes of infection-related hospitalization according to dialysis modality. Clin. J. Am. Soc. Nephrol. 10, 817–824 (2015).
Brown, E. A. et al. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis — position paper for ISPD: 2017 Update. Perit. Dial. Int. 37, 362–374 (2017).
Johnson, D. W. et al. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int. 77, 904–912 (2010).
Kawanishi, H. et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am. J. Kidney Dis. 44, 729–737 (2004).
Kawanishi, H., Shintaku, S., Banshodani, M. & Hashimoto, S. Past and present perspectives on encapsulating peritoneal sclerosis. Contrib. Nephrol. 185, 87–97 (2015).
Korte, M. R. et al. Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol. Dial. Transpl. 22, 2412–2414 (2007).
Singh, N. et al. Risk factors associated with peritoneal dialysis catheter survival: a 9-year single-center study in 315 patients. J. Vasc. Access. 11, 316–322 (2010).
Moreiras Plaza, M., Cuíña, L., Goyanes, G. R., Sobrado, J. A. & Gonzalez, L. Mechanical complications in chronic peritoneal dialysis. Clin. Nephrol. 52, 124–130 (1999).
Leblanc, M., Ouimet, D. & Pichette, V. Dialysate leaks in peritoneal dialysis. Semin. Dial. 14, 50–54 (2001).
Ye, H. et al. Urgent-start peritoneal dialysis for patients with end stage renal disease: a 10-year retrospective study. BMC Nephrol. 20, 238 (2019).
García Ramón, R. & Carrasco, A. M. Hydrothorax in peritoneal dialysis. Perit. Dial. Int. 18, 5–10 (1998).
Abraham, G., Shokker, A., Blake, P. & Oreopoulos, D. Massive hydrothorax in patients on peritoneal dialysis: a literature review. Adv. Perit. Dial. Conf. Perit. Dial. 4, 121 (1988).
Jazayeri-Moghadass, B. S., Sutherland, R., Patel, L. D. & Cebotaru, V. Small bowel obstruction with a transition point in a patient on peritoneal dialysis. Case Rep. Nephrol. Dial. 12, 6–10 (2022).
Kavanagh, N. T., Schiller, B., Saxena, A. B., Thomas, I. C. & Kurella Tamura, M. Prevalence and correlates of functional dependence among maintenance dialysis patients. Hemodial. Int. 19, 593–600 (2015).
Wilson, S. et al. Known unknowns: Examining the burden of neurocognitive impairment in the end-stage renal failure population. Nephrology 23, 501–506 (2018).
Black, N. Patient reported outcome measures could help transform healthcare. BMJ 346, f167 (2013).
Kalantar-Zadeh, K. et al. Living Well With Kidney Disease by Patient and Care-Partner Empowerment: Kidney Health for Everyone Everywhere. Can. J. Kidney Health Dis. 8, 2054358121995276 (2021).
Okpechi, I. G., Nthite, T. & Swanepoel, C. R. Health-related quality of life in patients on hemodialysis and peritoneal dialysis. Saudi J. Kidney Dis. Transplant. 24, 519 (2013).
Purnell, T. S. et al. Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am. J. Kidney Dis. 62, 953–973 (2013).
Molsted, S., Prescott, L., Heaf, J. & Eidemak, I. Assessment and clinical aspects of health-related quality of life in dialysis patients and patients with chronic kidney disease. Nephron. Clin. Pract. 106, c24–c33 (2007).
Julius, M. et al. A comparison of employment rates of patients treated with continuous ambulatory peritoneal dialysis vs in-center hemodialysis (Michigan End-Stage Renal Disease Study). Arch. Intern. Med. 149, 839–842 (1989).
Chuasuwan, A., Pooripussarakul, S., Thakkinstian, A., Ingsathit, A. & Pattanaprateep, O. Comparisons of quality of life between patients underwent peritoneal dialysis and hemodialysis: a systematic review and meta-analysis. Health Qual. Life Outcomes 18, 191 (2020).
Zazzeroni, L., Pasquinelli, G., Nanni, E., Cremonini, V. & Rubbi, I. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press. Res. 42, 717–727 (2017).
Raoofi, S. et al. Hemodialysis and peritoneal dialysis-health-related quality of life: systematic review plus meta-analysis. BMJ Support. Palliat. Care https://doi.org/10.1136/bmjspcare-2021-003182 (2021).
Ghani, Z., Rydell, H. & Jarl, J. The effect of peritoneal dialysis on labor market outcomes compared with institutional hemodialysis. Perit. Dial. Int. 39, 59–65 (2019).
Fan, L. et al. Burden of kidney disease among patients with peritoneal dialysis versus conventional in-centre haemodialysis: a randomised, non-inferiority trial. Perit. Dial. Int. 42, 246–258 (2022).
Bunchman, T. E. & Ballal, S. H. Treatment of inflow pain by pH adjustment of dialysate in peritoneal dialysis. Perit. Dial. Int. 11, 179–180 (1991).
Htay, H. et al. Biocompatible dialysis fluids for peritoneal dialysis. Cochrane Database Syst. Rev. 10, CD007554 (2018).
Blake, P. G., Sloand, J. A., McMurray, S., Jain, A. K. & Matthews, S. A multicenter survey of why and how tidal peritoneal dialysis (TPD) is being used. Perit. Perit. Dial. Int 34, 458–460 (2014).
Yi, C. et al. The incidence of pain and its association with quality of life in patients with peritoneal dialysis. Ren. Fail. 44, 724–730 (2022).
Zuvela, J. et al. Gastrointestinal symptoms in patients receiving dialysis: a systematic review. Nephrology 23, 718–727 (2018).
Kosmadakis, G., Albaret, J., Da Costa Correia, E., Somda, F. & Aguilera, D. Constipation in peritoneal dialysis patients. Perit. Perit. Dial. Int 39, 399–404 (2019).
Wu, M. J. et al. Colonic transit time in long-term dialysis patients. Am. J. Kidney Dis. 44, 322–327 (2004).
Yi, C., Wang, X., Ye, H., Lin, J. & Yang, X. Patient-reported gastrointestinal symptoms in patients with peritoneal dialysis: the prevalence, influence factors and association with quality of life. BMC Nephrol. 23, 99 (2022).
Dong, R., Guo, Z. Y., Ding, J. R., Zhou, Y. Y. & Wu, H. Gastrointestinal symptoms: a comparison between patients undergoing peritoneal dialysis and hemodialysis. World J. Gastroenterol. 20, 11370–11375 (2014).
Salamon, K., Woods, J., Paul, E. & Huggins, C. Peritoneal dialysis patients have higher prevalence of gastrointestinal symptoms than hemodialysis patients. J. Ren. Nutr. 23, 114–118 (2013).
Zheng, Z. H. et al. Bicarbonate-based peritoneal dialysis solution has less effect on ingestive behavior than lactate-based peritoneal dialysis solution. Perit. Dial. Int. 29, 656–663 (2009).
Van, V. et al. Influence of dialysate on gastric emptying time in peritoneal dialysis patients. Perit. Dial. Int. 22, 32–38 (2002).
Artom, M., Moss-Morris, R., Caskey, F. & Chilcot, J. Fatigue in advanced kidney disease. Kidney Int. 86, 497–505 (2014).
Yngman-Uhlin, P., Kjellsdotter, A., Uhlin, F. & Edéll-Gustafsson, U. Sleep quality, fatigue, and health-related quality of life in patients on initial peritoneal dialysis and multiple modalities after two years: a prospective study. Nephrol. Nurs. J. 46, 615–649 (2019).
Jhamb, M. et al. Correlates and outcomes of fatigue among incident dialysis patients. Clin. J. Am. Soc. Nephrol. 4, 1779–1786 (2009).
Ossareh, S. et al. Fatigue in chronic peritoneal dialysis patients. Int. Urol. Nephrol. 35, 535–541 (2003).
Maruyama, Y., Nakayama, M., Ueda, A., Miyazaki, M. & Yokoo, T. Comparisons of fatigue between dialysis modalities: A cross-sectional study. PLoS One 16, e0246890 (2021).
Bonner, A., Wellard, S. & Caltabiano, M. The impact of fatigue on daily activity in people with chronic kidney disease. J. Clin. Nurs. 19, 3006–3015 (2010).
Bonner, A., Wellard, S. & Caltabiano, M. Levels of fatigue in people with ESRD living in far North Queensland. J. Clin. Nurs. 17, 90–98 (2008).
Chang, W. K., Hung, K. Y., Huang, J. W., Wu, K. D. & Tsai, T. J. Chronic fatigue in long-term peritoneal dialysis patients. Am. J. Nephrol. 21, 479–485 (2001).
Chaudhary, K. Peritoneal dialysis drop-out: causes and prevention strategies. Int. J. Nephrol. 2011, 434608 (2011).
Australian New Zealand Clinical Trials Registry. https://anzctr.org.au/ACTRN12620000408987.aspx (2022).
Brown, E. A. et al. Burden of kidney disease, health-related quality of life, and employment among patients receiving peritoneal dialysis and in-center hemodialysis: findings from the DOPPS Program. Am. J. Kidney Dis. 78, 489–500.e1 (2021).
Bautovich, A., Katz, I., Smith, M., Loo, C. K. & Harvey, S. B. Depression and chronic kidney disease: a review for clinicians. Aust. N. Z. J. Psychiatry 48, 530–541 (2014).
Hedayati, S. S. & Finkelstein, F. O. Epidemiology, diagnosis, and management of depression in patients with CKD. Am. J. Kidney Dis. 54, 741–752 (2009).
Mahajan, S. et al. Analysis of depression and its effect on outcome among adult Indian peritoneal dialysis patients. Perit. Dial. Int. 27, 94–96 (2007).
Mok, M. M. Y. et al. A longitudinal study on the prevalence and risk factors for depression and anxiety, quality of life, and clinical outcomes in incident peritoneal dialysis patients. Perit. Dial. Int. 39, 74–82 (2019).
Lin, J. et al. The negative impact of depressive symptoms on patient and technique survival in peritoneal dialysis: a prospective cohort study. Int. Urol. Nephrol. 52, 2393–2401 (2020).
Goh, Z. S. & Griva, K. Anxiety and depression in patients with end-stage renal disease: impact and management challenges — a narrative review. Int. J. Nephrol. Renovasc. Dis. 11, 93–102 (2018).
Griva, K. et al. Predicting technique and patient survival over 12 months in peritoneal dialysis: the role of anxiety and depression. Int. Urol. Nephrol. 48, 791–796 (2016).
Jacquet, S. & Trinh, E. The potential burden of home dialysis on patients and caregivers: a narrative review. Can. J. Kidney Health Dis. 6, 2054358119893335 (2019).
Figueiredo, A. E. et al. Evaluation of physical symptoms in patients on peritoneal dialysis. Int. J. Nephrol. 2012, 305424 (2012).
Kobrin, S. M. & Berns, J. S. Quinine — a tonic too bitter for hemodialysis-associated muscle cramps? Semin. Dial. 20, 396–401 (2007).
Takahashi, A. The pathophysiology of leg cramping during dialysis and the use of carnitine in its treatment. Physiol. Rep. 9, e15114 (2021).
Tessari, G. et al. The impact of pruritus on the quality of life of patients undergoing dialysis: a single centre cohort study. J. Nephrol. 22, 241–248 (2009).
Min, J. W. et al. Comparison of uremic pruritus between patients undergoing hemodialysis and peritoneal dialysis. Kidney Res. Clin. Pract. 35, 107–113 (2016).
Combs, S. A., Teixeira, J. P. & Germain, M. J. Pruritus in kidney disease. Semin. Nephrol. 35, 383–391 (2015).
Kim, D. & Pollock, C. Epidemiology and burden of chronic kidney disease-associated pruritus. Clin. Kidney J. 14, i1–i7 (2021).
Wu, H. Y. et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: a prospective cohort study. PLoS One 13, e0203474 (2018).
Makar, M., Smyth, B. & Brennan, F. Chronic kidney disease-associated pruritus: a review. Kidney Blood Press. Res. 46, 659–669 (2021).
Allen, R. P. et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep. Med. 4, 101–119 (2003).
Novak, M., Mendelssohn, D., Shapiro, C. M. & Mucsi, I. Diagnosis and management of sleep apnea syndrome and restless legs syndrome in dialysis patients. Semin. Dial. 19, 210–216 (2006).
Kavanagh, D., Siddiqui, S. & Geddes, C. C. Restless legs syndrome in patients on dialysis. Am. J. Kidney Dis. 43, 763–771 (2004).
Zamponi, V. et al. Association between type 1 diabetes and female sexual dysfunction. BMC Women’s Health 20, 73 (2020).
Navaneethan, S. D. et al. Prevalence and correlates of self-reported sexual dysfunction in CKD: a meta-analysis of observational studies. Am. J. Kidney Dis. 56, 670–685 (2010).
Guney, I. et al. Comparison of effects of automated peritoneal dialysis and continuous ambulatory peritoneal dialysis on health-related quality of life, sleep quality, and depression. Hemodial. Int. 14, 515–522 (2010).
Eryavuz, N. et al. Comparison of sleep quality between hemodialysis and peritoneal dialysis patients. Int. Urol. Nephrol. 40, 785–791 (2008).
Güney, I. et al. Sleep quality and depression in peritoneal dialysis patients. Ren. Fail. 30, 1017–1022 (2008).
Masoumi, M., Naini, A. E., Aghaghazvini, R., Amra, B. & Gholamrezaei, A. Sleep quality in patients on maintenance hemodialysis and peritoneal dialysis. Int. J. Prev. Med. 4, 165–172 (2013).
Stepanski, E., Faber, M., Zorick, F., Basner, R. & Roth, T. Sleep disorders in patients on continuous ambulatory peritoneal dialysis. J. Am. Soc. Nephrol. 6, 192–197 (1995).
Yang, J. Y. et al. Quality of sleep and psychosocial factors for patients undergoing peritoneal dialysis. Perit. Dial. Int 27, 675–680 (2007).
Li, J. et al. Prevalence and risk factors of sleep disturbance in continuous ambulatory peritoneal dialysis patients in Guangzhou, southern China. Int. Urol. Nephrol. 44, 929–936 (2012).
Buysse, D. J., Reynolds, C. F. 3rd, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr. Res. 28, 193–213 (1989).
Fleming, T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled. Ann. Intern. Med. 125, 605–613 (1996).
Bargman, J. M., Thorpe, K. E. & Churchill, D. N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J. Am. Soc. Nephrol. 12, 2158–2162 (2001).
Vonesh, E. F., Snyder, J. J., Foley, R. N. & Collins, A. J. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us? Kidney Int. 70 (Suppl. 103), S3–S11 (2006).
Cho, Y. et al. The impact of neutral-pH peritoneal dialysates with reduced glucose degradation products on clinical outcomes in peritoneal dialysis patients. Kidney Int. 84, 969–979 (2013).
Lee, Y. et al. Incremental peritoneal dialysis may be beneficial for preserving residual renal function compared to full-dose peritoneal dialysis. Sci. Rep. 9, 10105 (2019).
Htay, H. et al. Predictors of residual renal function decline in peritoneal dialysis patients: the balANZ Trial. Perit. Dial. Int. 37, 283–289 (2017).
Ethier, I. et al. Rate of decline in residual kidney function pre and post peritoneal dialysis initiation: a post hoc analysis of the IDEAL study. PLoS One 15, e0242254 (2020).
Woodrow, G. Volume status in peritoneal dialysis. Perit. Dial. Int. 31(Suppl 2), S77–S82 (2011).
Van Biesen, W. et al. Evolution over time of volume status and PD-related practice patterns in an incident peritoneal dialysis cohort. Clin. J. Am. Soc. Nephrol. 14, 882–893 (2019).
Tian, N. et al. Bioimpedance guided fluid management in peritoneal dialysis: a randomized controlled trial. Clin. J. Am. Soc. Nephrol. 15, 685–694 (2020).
Brown, E. A. et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit. Dial. Int. 40, 244–253 (2020).
Tan, B. K. et al. Longitudinal bioimpedance vector plots add little value to fluid management of peritoneal dialysis patients. Kidney Int. 89, 487–497 (2016).
Cocchi, R. et al. Prevalence of hypertension in patients on peritoneal dialysis: results of an Italian multicentre study. Nephrol. Dial. Transpl. 14, 1536–1540 (1999).
Koc, M. et al. Uncontrolled hypertension due to volume overload contributes to higher left ventricular mass index in CAPD patients. Nephrol. Dial. Transpl. 17, 1661–1666 (2002).
Menon, M. K., Naimark, D. M., Bargman, J. M., Vas, S. I. & Oreopoulos, D. G. Long-term blood pressure control in a cohort of peritoneal dialysis patients and its association with residual renal function. Nephrol. Dial. Transpl. 16, 2207–2213 (2001).
Jhee, J. H. et al. The optimal blood pressure target in different dialysis populations. Sci. Rep. 8, 14123 (2018).
Goldfarb-Rumyantzev, A. S., Baird, B. C., Leypoldt, J. K. & Cheung, A. K. The association between BP and mortality in patients on chronic peritoneal dialysis. Nephrol. Dial. Transpl. 20, 1693–1701 (2005).
Udayaraj, U. P. et al. Blood pressure and mortality risk on peritoneal dialysis. Am. J. Kidney Dis. 53, 70–78 (2009).
Wang, X., Axelsson, J., Lindholm, B. & Wang, T. Volume status and blood pressure in continuous ambulatory peritoneal dialysis patients. Blood Purif. 23, 373–378 (2005).
Li, P. K., Chow, K. M., Wong, T. Y., Leung, C. B. & Szeto, C. C. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann. Intern. Med. 139, 105–112 (2003).
Eckardt, K.-U. & Kasiske, B. L. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 76 (Suppl. 113), S1–S2 (2009).
Wang, A. Y. Vascular and other tissue calcification in peritoneal dialysis patients. Perit. Dial. Int. 29 (Suppl 2), S9–s14 (2009).
Levin, A. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 71, 31–38 (2007).
Tentori, F. et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS. Am. J. Kidney Dis. 52, 519–530 (2008).
Nitta, K., Hanafusa, N. & Tsuchiya, K. Mineral bone disorders (MBD) in patients on peritoneal dialysis. Ren. Replac. Ther. 5, 1–6 (2019).
Soohoo, M. et al. Comparative effectiveness of dialysis modality on laboratory parameters of mineral metabolism. Am. J. Nephrol. 53, 157–168 (2022).
Liu, C. T. et al. Roles of serum calcium, phosphorus, PTH and ALP on mortality in peritoneal dialysis patients: a nationwide, population-based longitudinal study using TWRDS 2005–2012. Sci. Rep. 7, 33 (2017).
Wu, M. et al. Associations between serum mineral metabolism parameters and mortality in patients on peritoneal dialysis. Nephrology 24, 1148–1156 (2019).
National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am. J. Kidney 47, S11–S145 (2006).
Wetmore, J. B. et al. Trends in anemia management practices in patients receiving hemodialysis and peritoneal dialysis: a retrospective cohort analysis. Am. J. Nephrol. 41, 354–361 (2015).
Li, P. K. T. et al. Anemia Management in Peritoneal Dialysis: Perspectives From the Asia Pacific Region. Kidney Med. 3, 405–411 (2021).
Wu, T. et al. Efficacy of roxadustat on anemia and residual renal function in patients new to peritoneal dialysis. Ren. Fail. 44, 529–540 (2022).
Oveyssi, J. et al. Patient and caregiver perspectives on burnout in peritoneal dialysis. Perit. Dial. Int. 41, 484–493 (2021).
Dahlerus, C. et al. Patient perspectives on the choice of dialysis modality: results from the empowering patients on choices for renal replacement therapy (EPOCH-RRT) Study. Am. J. Kidney Dis. 68, 901–910 (2016).
Brown, E. A., Dratwa, M. & Povlsen, J. V. Assisted peritoneal dialysis-an evolving dialysis modality. Nephrol. Dial. Transpl. 22, 3091–3092 (2007).
Sukul, N. et al. Patient-reported advantages and disadvantages of peritoneal dialysis: results from the PDOPPS. BMC Nephrol. 20, 116 (2019).
Subramanian, L. et al. Remote management for peritoneal dialysis: a qualitative study of patient, care partner, and clinician perceptions and priorities in the United States and the United Kingdom. Kidney Med. 1, 354–365 (2019).
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13, 393–409 (2017).
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982 (2015).
Yeung, E. et al. Current status of health systems financing and oversight for end-stage kidney disease care: a cross-sectional global survey. BMJ Open. 11, e047245 (2021).
Yang, F., Liao, M., Wang, P., Yang, Z. & Liu, Y. The cost-effectiveness of kidney replacement therapy modalities: a systematic review of full economic evaluations. Appl. Health Econ. Health Policy 19, 163–180 (2021).
Liu, J. et al. Financial implications of dialysis modalities in the developing world: s Chinese perspective. Perit. Dial. Int. 40, 193–201 (2020).
Schaefer, F. & Warady, B. A. Peritoneal dialysis in children with end-stage renal disease. Nat. Rev. Nephrol. 7, 659–668 (2011).
Driollet, B. et al. Social deprivation is associated with lower access to pre-emptive kidney transplantation and more urgent-start dialysis in the pediatric population. Kidney Int. Rep. 7, 741–751 (2022).
Nourse, P. et al. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 Update (paediatrics). Perit. Dial. Int. 41, 139–157 (2021).
Vidal, E. et al. Peritoneal dialysis in infants: the experience of the Italian Registry of Paediatric Chronic Dialysis. Nephrol. Dial. Transpl. 27, 388–395 (2012).
North American Pediatric Renal Trials and Collaborative Studies. 2011 Annual Dialysis Report, https://naprtcs.org/system/files/2011_Annual_Dialysis_Report.pdf (2011).
Carey, W. A., Martz, K. L. & Warady, B. A. Outcome of patients initiating chronic peritoneal dialysis during the first year of life. Pediatrics 136, e615–e622 (2015).
Verrina, E. et al. A multicenter experience on patient and technique survival in children on chronic dialysis. Pediatr. Nephrol. 19, 82–90 (2004).
Ploos van Amstel, S. et al. Mortality in children treated with maintenance peritoneal dialysis: findings from the International Pediatric Peritoneal Dialysis Network Registry. Am. J. Kidney Dis. 78, 380–390 (2021).
Prasad, N. et al. Long-term outcomes in children on chronic continuous ambulatory peritoneal dialysis: a retrospective cohort study from a developing country. Pediatr. Nephrol. 34, 2389–2397 (2019).
Bonthuis, M., Harambat, J., Jager, K. J. & Vidal, E. Growth in children on kidney replacement therapy: a review of data from patient registries. Pediatr. Nephrol. 36, 2563–2574 (2021).
System, U. S. R. D. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD).
Ros, S. et al. Increased risk of fatal infections in women starting peritoneal dialysis. Perit. Dial. Int. 33, 487–494 (2013).
Kotsanas, D., Polkinghorne, K. R., Korman, T. M., Atkins, R. C. & Brown, F. Risk factors for peritoneal dialysis-related peritonitis: can we reduce the incidence and improve patient selection. Nephrology 12, 239–245 (2007).
Kitterer, D., Segerer, S., Braun, N., Alscher, M. D. & Latus, J. Gender-specific differences in peritoneal dialysis. Kidney Blood Press. Res. 42, 276–283 (2017).
Shen, J. I., Mitani, A. A., Saxena, A. B., Goldstein, B. A. & Winkelmayer, W. C. Determinants of peritoneal dialysis technique failure in incident US patients. Perit. Dial. Int. 33, 155–166 (2013).
Lim, W. H., Dogra, G. K., McDonald, S. P., Brown, F. G. & Johnson, D. W. Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit. Dial. Int. 31, 663–671 (2011).
Mujais, S. & Story, K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int. 70 (Suppl. 103), S21–S26 (2006).
Yang, L. Y., Thia, E. W. & Tan, L. K. Obstetric outcomes in women with end-stage renal disease on chronic dialysis: a review. Obstet. Med. 3, 48–53 (2010).
McDonald, S. P. End-stage kidney disease among indigenous peoples of Australia and New Zealand. Kidney Int. Suppl. 3, 170–173 (2013).
Tonelli, M. et al. Use and outcomes of peritoneal dialysis among Aboriginal people in Canada. J. Am. Soc. Nephrol. 16, 482–488 (2005).
Prakash, S. An international perspective on peritoneal dialysis among indigenous patients. Perit. Dial. Int. 31, 390–398 (2011).
Lim, W. H. Is there a role for peritoneal dialysis in remote aboriginal patients with end-stage renal disease in Australia? Nephrology 9 (Suppl 4), S126–S128 (2004).
Lim, W. H., Johnson, D. W. & McDonald, S. P. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology 10, 192–197 (2005).
Lim, W. H. et al. Remote indigenous peritoneal dialysis patients have higher risk of peritonitis, technique failure, all-cause and peritonitis-related mortality. Nephrol. Dial. Transpl. 26, 3366–3372 (2011).
Ethier, I. et al. Dialysis initiation in older persons across centres and over time in Australia and New Zealand. Nephrology 26, 613–622 (2021).
Jiang, C. & Zheng, Q. Outcomes of peritoneal dialysis in elderly vs non-elderly patients: A systemic review and meta-analysis. PLoS One 17, e0263534 (2022).
Iyasere, O. et al. Longitudinal trends in quality of life and physical function in frail older dialysis patients: a comparison of assisted peritoneal dialysis and in-center hemodialysis. Perit. Dial. Int. 39, 112–118 (2019).
Hofmeister, M., Klarenbach, S., Soril, L., Scott-Douglas, N. & Clement, F. A systematic review and jurisdictional scan of the evidence characterizing and evaluating assisted peritoneal dialysis models. Clin. J. Am. Soc. Nephrol. 15, 511–520 (2020).
Maierean, S. M. & Oliver, M. J. Health outcomes and cost considerations of assisted peritoneal dialysis: a narrative review. Blood Purif. 50, 662–666 (2021).
Béchade, C. et al. Centre characteristics associated with the risk of peritonitis in peritoneal dialysis: a hierarchical modelling approach based on the data of the French Language Peritoneal Dialysis Registry. Nephrol. Dial. Transpl. 32, 1018–1023 (2017).
Ethier, I. et al. Effect of patient- and center-level characteristics on uptake of home dialysis in Australia and New Zealand: a multicenter registry analysis. Nephrol. Dial. Transpl. 35, 1938–1949 (2020).
Nadeau-Fredette, A. C. et al. Center-specific factors associated with peritonitis risk — a multi-center registry analysis. Perit. Dial. Int. 36, 509–518 (2016).
Htay, H. et al. Multicenter registry analysis of center characteristics associated with technique failure in patients on incident peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 12, 1090–1099 (2017).
Acknowledgements
D.W.J. is a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant. We thank Ghenette Houston and Sophanny Tiv of Kidney Health Research Group, University of Alberta for administrative and technical support.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content and wrote, reviewed, or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
V.J. has received fees from AstraZeneca, NephroPlus and Zydus Cadilla, and grants from Baxter Healthcare, Biocon and GlaxoSmithKline; all funds are paid to his organization. D.W.J. has received consultancy fees, research grants, speaker’s honoraria and travel sponsorships from Baxter Healthcare and Fresenius Medical Care, consultancy fees from Astra Zeneca, Bayer and AWAK, speaker’s honoraria from ONO and BI & Lilly, and travel sponsorships from Ono and Amgen. A.K.B. has received consultancy fees from Amgen, GlaxoSmithKline, Bayer and Otsuka. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks E. Brown and X. Yang for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Standardized Outcomes in Nephrology in PD (SONG-PD): https://songinitiative.org/projects/song-pd/
Supplementary information
Glossary
- Biocompatible PD solutions
-
PD solutions that have a neutral pH and relatively low concentrations of glucose degradation products.
- Centre effects
-
Variations in outcomes between centres that are related to centre characteristics (for example, practices, experiences and/or organization) rather than patient characteristics.
- PD vintage
-
The length of time (measured in months or years) during which a patient with kidney failure receives PD as a treatment modality.
- Executive function
-
A set of mental processes that enable people to plan, focus attention, retain and process information, and handle complex tasks.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bello, A.K., Okpechi, I.G., Osman, M.A. et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol 18, 779–793 (2022). https://doi.org/10.1038/s41581-022-00623-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-022-00623-7
This article is cited by
-
Prognostic value of transthoracic echocardiography score for the prognosis of continuous ambulatory peritoneal dialysis patients
BMC Nephrology (2024)
-
SGLT2 inhibitors in peritoneal dialysis: a promising frontier toward improved patient outcomes
Renal Replacement Therapy (2024)
-
The Intersectoral Coordination Unit for the Sustainable Intensification of Peritoneal Dialysis in Schleswig–Holstein (SKIP-SH) cohort study
BMC Nephrology (2024)
-
A new era in the science and care of kidney diseases
Nature Reviews Nephrology (2024)
-
Development of a clinical risk score system for peritoneal dialysis-associated peritonitis treatment failure
BMC Nephrology (2023)