Abstract
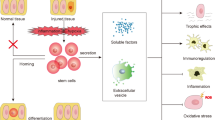
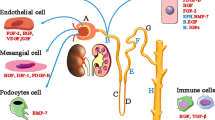
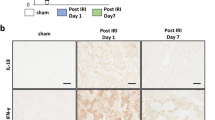
Acute kidney injury (AKI) is a common clinical complication, associated with poor outcomes and the development of chronic kidney disease. Despite major advances in the understanding of its pathophysiology, available therapies for AKI are only supportive; therefore, adequate functional recovery from AKI must predominantly rely on the kidney's own reparative ability. An extensive body of preclinical data from our own and from other laboratories has shown that administration of adult multipotent marrow stromal cells (commonly referred to as mesenchymal stem cells [MSCs]), effectively ameliorates experimental AKI by exerting paracrine renoprotective effects and by stimulating tissue repair. Based on these findings, a clinical trial has been conducted to investigate the safety and efficacy of MSCs administered to open-heart surgery patients who are at high risk of postoperative AKI. In this Perspectives article, we discuss some of the early data from this trial and describe potential applications for stem cell therapies in other fields of nephrology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Waikar, S. S., Liu, K. D. & Chertow, G. M. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin. J. Am. Soc. Nephrol. 3, 844–861 (2008).
Macedo, E., Bouchard, J. & Mehta, R. L. Renal recovery following acute kidney injury. Curr. Opin. Crit. Care 14, 660–665 (2008).
Kelly, K. J. & Molitoris, B. A. Acute renal failure in the new millennium: time to consider combination therapy. Semin. Nephrol. 20, 4–19 (2000).
Chertow, G. M. On the design and analysis of multicenter trials in acute renal failure. Am. J. Kidney Dis. 30 (Suppl. 4), S96–S101 (1997).
Hirschberg, R. et al. Multicenter clinical trial of recombinant human insulin-like growth factor I in patients with acute renal failure. Kidney Int. 55, 2423–2432 (1999).
Waikar, S. S. & Bonventre, J. V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 20, 672–679 (2009).
Coca, S. G., Yalavarthy, R., Concato, J. & Parikh, C. R. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 73, 1008–1016 (2008).
Vaidya, V. S. et al. A rapid urine test for early detection of kidney injury. Kidney Int. 76, 108–114 (2009).
Gnecchi, M., Zhang, Z., Ni, A. & Dzau, V. J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219 (2008).
Phinney, D. G. & Prockop, D. J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 25, 2896–2902 (2007).
Tögel, F., Zhang, P., Hu, Z. & Westenfelder, C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J. Cell. Mol. Med. 13, 2109–2114 (2009).
Tögel, F. et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Renal Physiol. 292, F1626–F1635 (2007).
Morigi, M. et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J. Am. Soc. Nephrol. 15, 1794–1804 (2004).
Tögel, F. et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Renal Physiol. 289, F31–F42 (2005).
Lange, C. et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 68, 1613–1617 (2005).
Tögel, F. et al. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 18, 475–485 (2009).
Duffield, J. S. et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Invest. 115, 1743–1755 (2005).
Bruno, S. et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 (2009).
Imberti, B. et al. Insulin-like growth factor-1 sustains stem cell mediated renal repair. J. Am. Soc. Nephrol. 18, 2921–2928 (2007).
Sheridan, A. M. & Bonventre, J. V. Cell biology and molecular mechanisms of injury in ischemic acute renal failure. Curr. Opin. Nephrol. Hypertens. 9, 427–434 (2000).
Giordano, A., Galderisi, U. & Marino, I. R. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J. Cell. Physiol. 211, 27–35 (2007).
US Clinical trials website [online], (2009).
US Clinical Trials Allogeneic Multipotent Stromal Cell Treatment for Acute Kidney Injury Following Cardiac Surgery [online], (2009).
Gooch, A. et al. Initial report on a phase I clinical trial: prevention and treatment of post-operative acute kidney injury with allogeneic mesenchymal stem cells in patients who require on-pump cardiac surgery. Cellular Therapy and Transplantation [online], (2008).
Koç, O. N. et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J. Clin. Oncol. 18, 307–316 (2000).
Le Blanc, K. et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371, 1579–1586 (2008).
Acknowledgements
The authors' research discussed in this Perspectives article was in part supported by funds from the Merit Review program of the Veterans Administration, Washington, DC, the NIH, the American Heart Association, the National Kidney Foundation, the Western Institute for Biomedical Research, and Allocure, Inc. The human MSCs that were administered to study subjects in the phase I clinical trial were prepared in the cGMP Cell Therapy Facility of the University of Utah, UT, USA. The outstanding skills of the principal investigators (J. Doty, Intermountain Medical Center, Murray, UT; D. Affleck, St. Mark's Hospital, Salt Lake City, UT), co-principal investigators (B. Horne and B. Muhlestein, Intermountain Medical Center; S. Karwande and G. Schorlemmer, St. Mark's Hospital), and study nurses (J. Flores, Intermountain Medical Center; A. Creer, St. Mark's Hospital) are gratefully acknowledged. The contributions of the members of the Data Safety and Monitoring Board for the phase I trial discussed in this article, C. Kablitz, G. R. Reiss, and S. Beddhu, are also greatly appreciated. Finally, the excellent bench work of Z. Hu and P. Zhang for the conduct of the described preclinical studies is acknowledged, and the regulatory contributions of A. Gooch were invaluable for the conduct of the described clinical trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C. Westenfelder has acted as a consultant, received grant/research support from and is a patent holder/applicant for Allocure. F.E. Tögel declares no competing interests.
Rights and permissions
About this article
Cite this article
Tögel, F., Westenfelder, C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol 6, 179–183 (2010). https://doi.org/10.1038/nrneph.2009.229
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.229
This article is cited by
-
Cell and gene therapy for kidney disease
Nature Reviews Nephrology (2023)
-
Electric field-directed migration of mesenchymal stem cells enhances their therapeutic potential on cisplatin-induced acute nephrotoxicity in rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Extracellular Vesicles Derived from Mesenchymal Stem Cells: A Potential Biodrug for Acute Respiratory Distress Syndrome Treatment
BioDrugs (2022)
-
Stem cells in the treatment of renal fibrosis: a review of preclinical and clinical studies of renal fibrosis pathogenesis
Stem Cell Research & Therapy (2021)
-
Stem/progenitor cell in kidney: characteristics, homing, coordination, and maintenance
Stem Cell Research & Therapy (2021)