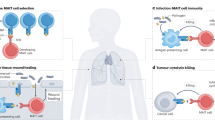
Abstract
Mucosal-associated invariant T (MAIT) cells are a unique T cell subset in mammals. They are present at high frequencies at mucosal tissue sites and have an intrinsic capacity to respond to microbial infections. The semi-invariant antigen recognition receptor of MAIT cells detects the non-polymorphic antigen-presenting molecule major histocompatibility complex class I-related protein 1 (MR1), which can bind microorganism-derived riboflavin metabolites. The striking evolutionary conservation in both the MR1 molecule and the MAIT T cell receptor suggests that strong selective pressures maintain this T cell pattern recognition system which detects microbial infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pancer, Z. & Cooper, M. D. The evolution of adaptive immunity. Annu. Rev. Immunol. 24, 497–518 (2006).
Medzhitov, R. & Janeway, C. A. Jr. Decoding the patterns of self and nonself by the innate immune system. Science 296, 298–300 (2002).
Kaufman, J. Evolution and immunity. Immunology 130, 459–462 (2010).
Litman, G. W., Rast, J. P. & Fugmann, S. D. The origins of vertebrate adaptive immunity. Nature Rev. Immunol. 10, 543–553 (2010).
Boehm, T. & Bleul, C. C. The evolutionary history of lymphoid organs. Nature Immunol. 8, 131–135 (2007).
Obar, J. J. & Lefrancois, L. Memory CD8+ T cell differentiation. Ann. N. Y. Acad. Sci. 1183, 251–266 (2010).
Weng, N. P., Araki, Y. & Subedi, K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nature Rev. Immunol. 12, 306–315 (2012).
Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 178, 1–16 (1993).
Tilloy, F. et al. An invariant T cell receptor α chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted α/β T cell subpopulation in mammals. J. Exp. Med. 189, 1907–1921 (1999).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, e1000407 (2010).
Benlagha, K., Kyin, T., Beavis, A., Teyton, L. & Bendelac, A. A thymic precursor to the NK T cell lineage. Science 296, 553–555 (2002).
Bendelac, A., Savage, P. B. & Teyton, L. The biology of NKT cells. Annu. Rev. Immunol. 25, 297–336 (2007).
Gold, M. C. et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 13 Jun 2012 (doi:10.1038/mi.2012.45).
Martin, E. et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 7, e54 (2009).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nature Immunol. 11, 701–708 (2010).
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 10 Oct 2012 (doi:10.1038/nature11605).
Miyazaki, Y., Miyake, S., Chiba, A., Lantz, O. & Yamamura, T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int. Immunol. 23, 529–535 (2011).
Goldfinch, N. et al. Conservation of mucosal associated invariant T (MAIT) cells and the MR1 restriction element in ruminants, and abundance of MAIT cells in spleen. Vet. Res. 41, 62 (2010).
Gold, M. C. et al. Human innate Mycobacterium tuberculosis-reactive αβTCR+ thymocytes. PLoS Pathog. 4, e39 (2008).
Lewinsohn, D. M., Briden, A. L., Reed, S. G., Grabstein, K. H. & Alderson, M. R. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 165, 925–930 (2000).
Lewinsohn, D. M. et al. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J. Exp. Med. 187, 1633–1640 (1998).
Lewinsohn, D. M. et al. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J. Immunol. 166, 439–446 (2001).
Lewinsohn, D. A., Gold, M. C. & Lewinsohn, D. M. Views of immunology: effector T cells. Immunol. Rev. 240, 25–39 (2011).
Georgel, P., Radosavljevic, M., Macquin, C. & Bahram, S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol. Immunol. 48, 769–775 (2011).
Chua, W. J. et al. Polyclonal MAIT cells have unique innate functions in bacterial infection. Infect. Immun. 80, 3256–3267 (2012).
Smith, K., McCoy, K. D. & Macpherson, A. J. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 19, 59–69 (2007).
Riegert, P., Wanner, V. & Bahram, S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J. Immunol. 161, 4066–4077 (1998).
Miley, M. J. et al. Biochemical features of the MHC-related protein 1 consistent with an immunological function. J. Immunol. 170, 6090–6098 (2003).
Huang, S. et al. MR1 uses an endocytic pathway to activate mucosal-associated invariant T cells. J. Exp. Med. 205, 1201–1211 (2008).
Yamaguchi, H., Kurosawa, Y. & Hashimoto, K. Expanded genomic organization of conserved mammalian MHC class I-related genes, human MR1 and its murine ortholog. Biochem. Biophys. Res. Commun. 250, 558–564 (1998).
Huang, S. et al. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc. Natl Acad. Sci. USA 106, 8290–8295 (2009).
Chua, W. J. et al. Endogenous MHC-related protein 1 is transiently expressed on the plasma membrane in a conformation that activates mucosal-associated invariant T cells. J. Immunol. 186, 4744–4750 (2011).
Greenaway, H. Y. et al. NKT and MAIT invariant TCRα sequences can be produced efficiently by VJ gene recombination. Immunobiology 1 May 2012 (doi:10.1016/j.imbio.2012.04.003).
Coles, M. C. & Raulet, D. H. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J. Immunol. 164, 2412–2418 (2000).
Reantragoon, R. et al. Structural insight into MR1-mediated recognition of the mucosal associated invariant T cell receptor. J. Exp. Med. 209, 761–774 (2012).
Scott-Browne, J. P. et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nature Immunol. 8, 1105–1113 (2007).
Borg, N. A. et al. CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448, 44–49 (2007).
Mallevaey, T. et al. T cell receptor CDR2β and CDR3β loops collaborate functionally to shape the iNKT cell repertoire. Immunity 31, 60–71 (2009).
Matulis, G. et al. Innate-like control of human iNKT cell autoreactivity via the hypervariable CDR3β loop. PLoS Biol. 8, e1000402 (2010).
Croxford, J. L., Miyake, S., Huang, Y. Y., Shimamura, M. & Yamamura, T. Invariant Vα19i T cells regulate autoimmune inflammation. Nature Immunol. 7, 987–994 (2006).
Miller, M. M. et al. Characterization of two avian MHC-like genes reveals an ancient origin of the CD1 family. Proc. Natl Acad. Sci. USA 102, 8674–8679 (2005).
Salomonsen, J. et al. Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc. Natl Acad. Sci. USA 102, 8668–8673 (2005).
Hee, C. S. et al. Structure of a classical MHC class I molecule that binds “non-classical” ligands. PLoS Biol. 8, e1000557 (2010).
Kaech, S. M., Hemby, S., Kersh, E. & Ahmed, R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111, 837–851 (2002).
Litman, G. W. & Cooper, M. D. Why study the evolution of immunity? Nature Immunol. 8, 547–548 (2007).
Marrack, P., Scott-Browne, J. P., Dai, S., Gapin, L. & Kappler, J. W. Evolutionarily conserved amino acids that control TCR-MHC interaction. Annu. Rev. Immunol. 26, 171–203 (2008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Gold, M., Lewinsohn, D. Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Microbiol 11, 14–19 (2013). https://doi.org/10.1038/nrmicro2918
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2918
This article is cited by
-
Francisella tularensis induces Th1 like MAIT cells conferring protection against systemic and local infection
Nature Communications (2021)
-
TRAV1-2+ CD8+ T-cells including oligoconal expansions of MAIT cells are enriched in the airways in human tuberculosis
Communications Biology (2019)
-
Enumeration, functional responses and cytotoxic capacity of MAIT cells in newly diagnosed and relapsed multiple myeloma
Scientific Reports (2018)
-
Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection
Scientific Reports (2016)
-
The intracellular pathway for the presentation of vitamin B–related antigens by the antigen-presenting molecule MR1
Nature Immunology (2016)