Abstract
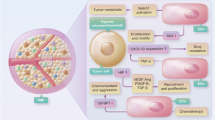
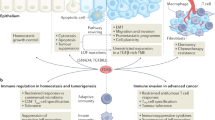
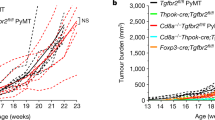
Emerging evidence indicates that angiogenesis and immunosuppression frequently occur simultaneously in response to diverse stimuli. Here, we describe a fundamental biological programme that involves the activation of both angiogenesis and immunosuppressive responses, often through the same cell types or soluble factors. We suggest that the initiation of these responses is part of a physiological and homeostatic tissue repair programme, which can be co-opted in pathological states, notably by tumours. This view can help to devise new cancer therapies and may have implications for aseptic tissue injury, pathogen-mediated tissue destruction, chronic inflammation and even reproduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Szekanecz, Z. & Koch, A. E. Mechanisms of disease: angiogenesis in inflammatory diseases. Nature Clin. Pract. Rheumatol. 3, 635–643 (2007).
Yang, L. et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 (2004).
Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev. Cancer 5, 263–274 (2005).
Riboldi, E. et al. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J. Immunol. 175, 2788–2792 (2005).
Hanna, J. et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nature Med. 12, 1065–1074 (2006).
Jablonska, J., Leschner, S., Westphal, K., Lienenklaus, S. & Weiss, S. Neutrophils responsive to endogenous IFN-β regulate tumor angiogenesis and growth in a mouse tumor model. J. Clin. Invest. 120, 1151–1164 (2010).
Shrestha, B. et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J. Immunol. 184, 4819–4826 (2010).
Qin, Z. et al. B cells inhibit induction of T cell-dependent tumor immunity. Nature Med. 4, 627–630 (1998).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via Ccl28 and TReg cells. Nature 475, 226–230 (2011).
Buckanovich, R. J. et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nature Med. 14, 28–36 (2008).
West, X. Z. et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467, 972–976 (2010).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Kandalaft, L. E., Powell, D. J. Jr, Singh, N. & Coukos, G. Immunotherapy for ovarian cancer: what's next? J. Clin. Oncol. 29, 925–933 (2010).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Rev. Immunol. 9, 162–174 (2009).
Gallina, G. et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116, 2777–2790 (2006).
Huang, B. et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66, 1123–1131 (2006).
Shojaei, F. et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 450, 825–831 (2007).
Murdoch, C., Muthana, M., Coffelt, S. B. & Lewis, C. E. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Rev. Cancer 8, 618–631 (2008).
Conejo-Garcia, J. R. et al. Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A. Nature Med. 10, 950–958 (2004).
Sica, A. et al. Autocrine production of IL-10 mediates defective IL-12 production and NF-κB activation in tumor-associated macrophages. J. Immunol. 164, 762–767 (2000).
Burke, B. et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am. J. Pathol. 163, 1233–1243 (2003).
Dirkx, A. E., Oude Egbrink, M. G., Wagstaff, J. & Griffioen, A. W. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J. Leukoc. Biol. 80, 1183–1196 (2006).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 10, 942–949 (2004).
Giatromanolaki, A. et al. The presence of tumor-infiltrating FOXP3+ lymphocytes correlates with intratumoral angiogenesis in endometrial cancer. Gynecol. Oncol. 110, 216–221 (2008).
Gupta, S., Joshi, K., Wig, J. D. & Arora, S. K. Intratumoral FOXP3 expression in infiltrating breast carcinoma: its association with clinicopathologic parameters and angiogenesis. Acta Oncol. 46, 792–797 (2007).
Freeman, M. R. et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 55, 4140–4145 (1995).
Stabile, E. et al. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 108, 205–210 (2003).
Huang, M. T. et al. Regulatory T cells negatively regulate neovasculature of airway remodeling via DLL4–Notch signaling. J. Immunol. 183, 4745–4754 (2009).
Bourbie-Vaudaine, S., Blanchard, N., Hivroz, C. & Romeo, P. H. Dendritic cells can turn CD4+ T lymphocytes into vascular endothelial growth factor-carrying cells by intercellular neuropilin-1 transfer. J. Immunol. 177, 1460–1469 (2006).
Sarris, M., Andersen, K. G., Randow, F., Mayr, L. & Betz, A. G. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413 (2008).
Inoue, S., Leitner, W. W., Golding, B. & Scott, D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 66, 7741–7747 (2006).
Halder, R. C., Aguilera, C., Maricic, I. & Kumar, V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J. Clin. Invest. 117, 2302–2312 (2007).
Lu, L. et al. Regulation of activated CD4+ T cells by NK cells via the Qa-1–NKG2A inhibitory pathway. Immunity 26, 593–604 (2007).
Wakita, D. et al. Tumor-infiltrating IL-17-producing γδT cells support the progression of tumor by promoting angiogenesis. Eur. J. Immunol. 40, 1927–1937 (2010).
Angeli, V. et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 (2006).
Kalkunte, S. S. et al. Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal–fetal interface. J. Immunol. 182, 4085–4092 (2009).
Kyriakakis, E. et al. Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. Eur. J. Immunol. 40, 3268–3279 (2010).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nature Rev. Cancer 6, 392–401 (2006).
Fukumura, D. et al. Tumor induction of VEGF promoter activity in stromal cells. Cell 94, 715–725 (1998).
Bottazzi, B., Walter, S., Govoni, D., Colotta, F. & Mantovani, A. Monocyte chemotactic cytokine gene transfer modulates macrophage infiltration, growth, and susceptibility to IL-2 therapy of a murine melanoma. J. Immunol. 148, 1280–1285 (1992).
Bhowmick, N. A., Neilson, E. G. & Moses, H. L. Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337 (2004).
da Silva Meirelles, L., Caplan, A. I. & Nardi, N. B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287–2299 (2008).
Krampera, M. et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood 101, 3722–3729 (2003).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 (2005).
Ringden, O. et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 81, 1390–1397 (2006).
Ellis, L. M. & Hicklin, D. J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev. Cancer 8, 579–591 (2008).
Della Porta, M. et al. Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology 68, 276–284 (2005).
Takahashi, A. et al. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol. Immunother. 53, 543–550 (2004).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nature Med. 2, 1096–1103 (1996).
Ishida, T., Oyama, T., Carbone, D. P. & Gabrilovich, D. I. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J. Immunol. 161, 4842–4851 (1998).
Curiel, T. J. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nature Med. 9, 562–567 (2003).
Ohm, J. E. et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101, 4878–4886 (2003).
Huang, Y. et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110, 624–631 (2007).
Shin, J. Y., Yoon, I. H., Kim, J. S., Kim, B. & Park, C. G. Vascular endothelial growth factor-induced chemotaxis and IL-10 from T cells. Cell. Immunol. 256, 72–78 (2009).
Li, B. et al. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin. Cancer Res. 12, 6808–6816 (2006).
Suzuki, H. et al. VEGFR2 is selectively expressed by FOXP3high CD4+ TReg . Eur. J. Immunol. 40, 197–203 (2010).
Montesinos, M. C., Shaw, J. P., Yee, H., Shamamian, P. & Cronstein, B. N. Adenosine A2A receptor activation promotes wound neovascularization by stimulating angiogenesis and vasculogenesis. Am. J. Pathol. 164, 1887–1892 (2004).
Alfranca, A. et al. PGE2 induces angiogenesis via MT1-MMP-mediated activation of the TGFβ/Alk5 signaling pathway. Blood 112, 1120–1128 (2008).
Lebrin, F., Deckers, M., Bertolino, P. & Ten Dijke, P. TGF-β receptor function in the endothelium. Cardiovasc. Res. 65, 599–608 (2005).
Zanin-Zhorov, A. et al. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J. Clin. Invest. 116, 2022–2032 (2006).
Manicassamy, S. et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature Med. 15, 401–409 (2009).
Yamazaki, S. et al. TLR2-dependent induction of IL-10 and Foxp3+CD25+CD4+ regulatory T cells prevents effective anti-tumor immunity induced by Pam2 lipopeptides in vivo. PLoS ONE 6, e18833 (2011).
Oldford, S. A. et al. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 185, 7067–7076 (2010).
Imanishi, T. et al. Cutting edge: TLR2 directly triggers Th1 effector functions. J. Immunol. 178, 6715–6719 (2007).
Chamorro, S. et al. TLR triggering on tolerogenic dendritic cells results in TLR2 up-regulation and a reduced proinflammatory immune program. J. Immunol. 183, 2984–2994 (2009).
Muller, W. A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 28, 323–344 (2011).
Bouma-ter Steege, J. C. et al. Angiogenic profile of breast carcinoma determines leukocyte infiltration. Clin. Cancer Res. 10, 7171–7178 (2004).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Detmar, M. et al. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J. Invest. Dermatol. 111, 1–6 (1998).
Griffioen, A. W., Damen, C. A., Blijham, G. H. & Groenewegen, G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 88, 667–673 (1996).
Griffioen, A. W., Damen, C. A., Martinotti, S., Blijham, G. H. & Groenewegen, G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 56, 1111–1117 (1996).
Bouzin, C., Brouet, A., De Vriese, J., Dewever, J. & Feron, O. Effects of vascular endothelial growth factor on the lymphocyte–endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J. Immunol. 178, 1505–1511 (2007).
Min, J. K. et al. Hepatocyte growth factor suppresses vascular endothelial growth factor-induced expression of endothelial ICAM-1 and VCAM-1 by inhibiting the nuclear factor-κB pathway. Circ. Res. 96, 300–307 (2005).
Dirkx, A. E. et al. Tumor angiogenesis modulates leukocyte–vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 63, 2322–2329 (2003).
Bagnato, A. et al. Expression of endothelin 1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res. 59, 720–727 (1999).
Nummer, D. et al. Role of tumor endothelium in CD4+ CD25+ regulatory T cell infiltration of human pancreatic carcinoma. J. Natl Cancer Inst. 99, 1188–1199 (2007).
Shetty, S. et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol. 186, 4147–4155 (2011).
Schenkel, A. R., Mamdouh, Z., Chen, X., Liebman, R. M. & Muller, W. A. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nature Immunol. 3, 143–150 (2002).
Mazanet, M. M. & Hughes, C. C. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169, 3581–3588 (2002).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Sata, M. & Walsh, K. TNFα regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nature Med. 4, 415–420 (1998).
Secchiero, P. & Zauli, G. The puzzling role of TRAIL in endothelial cell biology. Arterioscler. Thromb. Vasc. Biol. 28, e4; author reply e5–e6 (2008).
Ma, L. et al. Ig gene-like molecule CD31 plays a nonredundant role in the regulation of T-cell immunity and tolerance. Proc. Natl Acad. Sci. USA 107, 19461–19466 (2010).
Hernandez, G. L. et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J. Exp. Med. 193, 607–620 (2001).
Pirtskhalaishvili, G. & Nelson, J. B. Endothelium-derived factors as paracrine mediators of prostate cancer progression. Prostate 44, 77–87 (2000).
Huang, X. et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J. Exp. Med. 207, 505–520 (2010).
Batista, C. E. et al. Imaging correlates of differential expression of indoleamine 2,3-dioxygenase in human brain tumors. Mol. Imaging Biol. 11, 460–466 (2009).
Riesenberg, R. et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin. Cancer Res. 13, 6993–7002 (2007).
Wang, Y. et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nature Med. 16, 279–285 (2010).
Munn, D. H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Choi, J., Enis, D. R., Koh, K. P., Shiao, S. L. & Pober, J. S. T lymphocyte–endothelial cell interactions. Annu. Rev. Immunol. 22, 683–709 (2004).
Mulligan, J. K., Day, T. A., Gillespie, M. B., Rosenzweig, S. A. & Young, M. R. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum. Immunol. 70, 375–382 (2009).
Mulligan, J. K. & Young, M. R. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunol. Immunother. 59, 267–277 (2009).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Conejo-Garcia, J. R. et al. Vascular leukocytes contribute to tumor vascularization. Blood 105, 679–681 (2005).
De Palma, M., Venneri, M. A., Roca, C. & Naldini, L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nature Med. 9, 789–795 (2003).
Yan, S. F. et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J. Biol. Chem. 270, 11463–11471 (1995).
Cohen, T., Nahari, D., Cerem, L. W., Neufeld, G. & Levi, B. Z. Interleukin 6 induces the expression of vascular endothelial growth factor. J. Biol. Chem. 271, 736–741 (1996).
Wei, L. H. et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene 22, 1517–1527 (2003).
Kryczek, I. et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 203, 871–881 (2006).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Folkman, J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 285, 1182–1186 (1971).
Ivy, S. P., Wick, J. Y. & Kaufman, B. M. An overview of small-molecule inhibitors of VEGFR signaling. Nature Rev. Clin. Oncol. 6, 569–579 (2009).
Ebos, J. M. & Kerbel, R. S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nature Rev. Clin. Oncol. 8, 210–221 (2011).
Quezada, S. A. et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J. Exp. Med. 205, 2125–2138 (2008).
Chang, H. Y. et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc. Natl Acad. Sci. USA 102, 3738–3743 (2005).
Basu, G. D. et al. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J. Immunol. 177, 2391–2402 (2006).
Escudier, B. et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J. Clin. Oncol. 28, 2144–2150 (2010).
Rini, B. I. et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J. Clin. Oncol. 28, 2137–2143 (2010).
Chinnasamy, D. et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Invest. 120, 3953–3968 (2010).
Arenberg, D. A. et al. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J. Exp. Med. 184, 981–992 (1996).
Sgadari, C. et al. Mig, the monokine induced by interferon-γ, promotes tumor necrosis in vivo. Blood 89, 2635–2643 (1997).
Lasagni, L. et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 197, 1537–1549 (2003).
Yano, K. et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J. Exp. Med. 203, 1447–1458 (2006).
Cavassani, K. A. et al. The post sepsis-induced expansion and enhanced function of regulatory T cells create an environment to potentiate tumor growth. Blood 115, 4403–4411 (2010).
Pucci, F. et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood 114, 901–914 (2009).
Muthuswamy, R. et al. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 68, 5972–5978 (2008).
Baratelli, F. et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J. Immunol. 175, 1483–1490 (2005).
Ahmadi, M., Emery, D. C. & Morgan, D. J. Prevention of both direct and cross-priming of antitumor CD8+ T-cell responses following overproduction of prostaglandin E2 by tumor cells in vivo. Cancer Res. 68, 7520–7529 (2008).
Rodriguez, P. C. et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202, 931–939 (2005).
Ohta, A. et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J. Immunol. 183, 5487–5493 (2009).
Zarek, P. E. et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111, 251–259 (2008).
Hasko, G., Linden, J., Cronstein, B. & Pacher, P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nature Rev. Drug Discov. 7, 759–770 (2008).
Taylor, A. W. Review of the activation of TGF-β in immunity. J. Leukoc. Biol. 85, 29–33 (2009).
Li, Y. et al. Local expression of indoleamine 2,3-dioxygenase protects engraftment of xenogeneic skin substitute. J. Invest. Dermatol. 126, 128–136 (2006).
Biancone, L. et al. Development of inflammatory angiogenesis by local stimulation of Fas in vivo. J. Exp. Med. 186, 147–152 (1997).
Li, X. et al. A destructive cascade mediated by CCL2 facilitates prostate cancer growth in bone. Cancer Res. 69, 1685–1692 (2009).
Volpert, O. V. et al. Inhibition of angiogenesis by interleukin 4. J. Exp. Med. 188, 1039–1046 (1998).
Fukushi, J., Ono, M., Morikawa, W., Iwamoto, Y. & Kuwano, M. The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. J. Immunol. 165, 2818–2823 (2000).
Yamaji-Kegan, K., Su, Q., Angelini, D. J. & Johns, R. A. IL-4 is proangiogenic in the lung under hypoxic conditions. J. Immunol. 182, 5469–5476 (2009).
Eubank, T. D., Galloway, M., Montague, C. M., Waldman, W. J. & Marsh, C. B. M-CSF induces vascular endothelial growth factor production and angiogenic activity from human monocytes. J. Immunol. 171, 2637–2643 (2003).
Shojaei, F. et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc. Natl Acad. Sci. USA 106, 6742–6747 (2009).
Okazaki, T. et al. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int. Immunol. 18, 1–9 (2006).
Bronte, V. & Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nature Rev. Immunol. 5, 641–654 (2005).
Otsuji, M., Kimura, Y., Aoe, T., Okamoto, Y. & Saito, T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 ζ chain of T-cell receptor complex and antigen-specific T-cell responses. Proc. Natl Acad. Sci. USA 93, 13119–13124 (1996).
Spinella, F., Rosano, L., Di Castro, V., Natali, P. G. & Bagnato, A. Endothelin-1-induced prostaglandin E2-EP2, EP4 signaling regulates vascular endothelial growth factor production and ovarian carcinoma cell invasion. J. Biol. Chem. 279, 46700–46705 (2004).
Zou, W. et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature Med. 7, 1339–1346 (2001).
Lin, Y. L., Liang, Y. C. & Chiang, B. L. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J. Leukoc. Biol. 82, 1473–1480 (2007).
Fischer, C., Mazzone, M., Jonckx, B. & Carmeliet, P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nature Rev. Cancer 8, 942–956 (2008).
Sutmuller, R. P. et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116, 485–494 (2006).
Jarnicki, A. G. et al. Attenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeutics. J. Immunol. 180, 3797–3806 (2008).
Macedo, L. et al. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am. J. Pathol. 171, 1774–1788 (2007).
Wang, L. et al. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 206, 1457–1464 (2009).
Acknowledgements
The authors are supported by US National Institutes of Health grant R01-CA116779, US National Cancer Institute ovarian cancer SPORE grant P01-CA83638 and the Ovarian Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Adoptive cell therapy
-
Very simply, the transfusion of lymphocytes into patients for the treatment of cancer. Strategies that have enjoyed success include the rapid ex vivo expansion of tumour-infiltrating lymphocytes followed by autologous reinjection. Engineering of patient peripheral blood T cells to express artificial T cell receptors or chimeric antigen receptors that recognize tumour antigens is a recently developed strategy that has proved successful.
- Diapedesis
-
Leukocyte migration through the endothelium that is mediated by leukocyte-secreted proteases that disrupt the endothelial cell barrier.
- Extravasation
-
The multistep process of leukocyte infiltration through the endothelium. This process proceeds through the stages of leukocyte rolling, adhesion, diapedesis and finally migration to the surrounding tissues.
- Ischaemia–reperfusion injury
-
Disruption of proper blood flow, either experimentally or otherwise, followed by restoration of normal blood flow results in significant hypoxia, tissue damage and inflammation followed by angiogenesis and immunosuppression.
- Mural cells
-
Cells that physically surround the endothelial cells of blood vessels. This population is comprised of vascular smooth muscle cells (associated with veins and arteries) and pericytes (associated with capillaries and developing vessels).
- Pericyte
-
A mural cell that is thought to have significant roles in supporting the growth and survival of endothelial cells during angiogenesis, particularly in tumours.
- Trogocytosis
-
Following the formation of the immunological synapse, membrane fragments from the antigen-presenting cell are physically transferred to, and transiently incorporated in, the membrane of the interacting T cell (or B or NK cell). The biological significance remains wholly unknown.
Rights and permissions
About this article
Cite this article
Motz, G., Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 11, 702–711 (2011). https://doi.org/10.1038/nri3064
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3064
This article is cited by
-
Checkpoint inhibitors and anti-angiogenic agents: a winning combination
British Journal of Cancer (2023)
-
Immunobiology of high-grade serous ovarian cancer: lessons for clinical translation
Nature Reviews Cancer (2022)
-
Crosstalk between angiogenesis and immune regulation in the tumor microenvironment
Archives of Pharmacal Research (2022)
-
In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response
Nature Communications (2022)
-
Antiangiogenic antibody BD0801 combined with immune checkpoint inhibitors achieves synergistic antitumor activity and affects the tumor microenvironment
BMC Cancer (2021)