Abstract
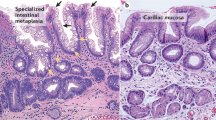
Barrett oesophagus develops when the lower oesophageal squamous epithelium is replaced with columnar epithelium, which shows both intestinal and gastric differentiation. No consensus has been reached on the origin of Barrett oesophagus. Theories include a direct origin from the oesophageal-stratified squamous epithelium, or by proximal migration of the gastric cardiac epithelium with subsequent intestinalization. Variations of this theory suggest the origin is a distinctive cell at the squamocolumnar junction, the oesophageal gland ducts, or circulating bone-marrow-derived cells. Much of the supporting evidence comes from experimental models and not from studies of Barrett mucosa. In this Perspectives article, we look at the Barrett lesion itself: at its phenotype, its complexity, its clonal architecture and its stem cell organization. We conclude that Barrett glands are unique structures, but share many similarities with gastric glands undergoing the process of intestinal metaplasia. We conclude that current evidence most strongly supports an origin from stem cells in the cardia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Haggitt, R., C. Adenocarcinoma in Barrett's esophagus: a new epidemic? Hum. Pathol. 23, 475–476 (1992).
Xian, W., Ho, K. Y., Crum, C. P. & McKeon, F. Cellular origin of Barrett's esophagus: controversy and therapeutic implications. Gastroenterology 142, 1424–1430 (2012).
Jass, J. R. Mucin histochemistry of the columnar epithelium of the oesophagus: a retrospective study. J. Clin. Pathol. 34, 866–870 (1981).
Liu, G. S. et al. Distinction between short-segment Barrett's esophageal and cardiac intestinal metaplasia. World J. Gastroenterol. 11, 6360–6365 (2005).
Hahn, H. P. et al. Intestinal differentiation in metaplastic, non-goblet columnar epithelium in the esophagus. Am. J. Surg. Pathol. 33, 1006–1015 (2009).
Spechler, S. J. Barrett's esophagus: is the goblet half empty? Clin. Gastroenterol. Hepatol. 10, 1237–1238 (2012).
Bansal, A. et al. Presence or absence of intestinal metaplasia but not its burden is associated with prevalent high-grade dysplasia and cancer in Barrett's esophagus. Dis. Esophagus http://dx.doi.org/10.1111/dote.12151.
Westerhoff, M., Hovan, L., Lee, C. & Hart, J. Effects of dropping the requirement for goblet cells from the diagnosis of Barrett's esophagus. Clin. Gastroenterol. Hepatol. 10, 1232–1236 (2012).
Riddell, R. H. & Odze, R. D. Definition of Barrett's esophagus: time for a rethink—is intestinal metaplasia dead? Am. J. Gastroenterol. 104, 2588–2594 (2009).
Fitzgerald, R. C. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 63, 7–42 (2014).
von Rahden, B. H., Stein, H. J. & Siewert, J. R. Barrett's esophagus and Barrett's carcinoma. Curr. Oncol. Rep. 5, 203–209 (2003).
Mahajan, D., Bennett, A. E., Liu, X., Bena, J. & Bronner, M. P. Grading of gastric foveolar-type dysplasia in Barrett's esophagus. Mod. Pathol. 23, 1–11 (2010).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012).
Goldblum, J. R. Barrett's esophagus and Barrett's-related dysplasia. Mod. Pathol. 16, 316–324 (2003).
Haggitt, R. C. Barrett's esophagus, dysplasia and adenocarcinoma. Hum. Pathol. 25, 982–993 (1994).
Weinstein, W. M. & Ippoliti, A. F. The diagnosis of Barrett's esophagus. Goblets, goblets, goblets. Gastrointest. Endosc. 44, 91–94 (1996).
Sampliner, R. E. Practice guidelines on the diagnosis, surveillance and therapy of Barrett's esophagus. Am. J. Gastroenterol. 93, 1028–1031 (1998).
Lee, R. G. Mucins in Barrett's esophagus: a histochemical study. Am. J. Clin. Pathol. 81, 500–503 (1984).
Chen, Y. Y. et al. Significance of acid-mucin-positive non-goblet columnar cells in the distal esophagus and gastroesophageal junction. Hum. Pathol. 30, 1488–1495 (1999).
Rouzier, R. & Robine, S. The subtleties of intestinal metaplasia. Gut 49, 8 (2001).
Duchatelle, V., Potet, F., Bara, J., Ma, J. & Goldfain, D. Mucin immunohistochemistry of the columnar epithelium of the oesophagus (Barrett's oesophagus). Virchows Arch. A Pathol. Anat. Histopathol. 414, 359–363 (1989).
Jauregui, H. O., Davessar, K., Hale, J. H. Kessimian, N. & Cenoz, C. Mucin histochemistry of intestinal metaplasia in Barrett's esophagus. Mod. Pathol. 1, 188–192 (1988).
Lapertosa, G., Baracchini, P. & Fulcheri, E. Mucin histochemical analysis in the interpretation of Barrett's esophagus. Results of a multicenter study. The Operative Group for the Study of Esophageal Precancer. Am. J. Clin. Pathol. 98, 61–66 (1992).
Torrado, J. et al. Blood-group phenotypes, sulfomucins, and Helicobacter pylori in Barrett's esophagus. Am. J. Surg. Pathol. 21, 1023–1029 (1997).
Glickman, J. N. et al. Mucin core polypeptide expression in the progression of neoplasia in Barrett's esophagus. Hum. Pathol. 37, 1304–1315 (2006).
Hanby, A. M., Jankowski, J. A., Elia, G. Poulsom, R. & Wright, N. A. Expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in Barrett's metaplasia and the native oesophageal epithelium: delineation of epithelial phenotype. J. Pathol. 173, 213–219 (1994).
Van De Bovenkamp, J. H. et al. Gastric-type mucin and TFF-peptide expression in Barrett's oesophagus is disturbed during increased expression of MUC2. Histopathology 42, 555–565 (2003).
Peitz, U. et al. TFF3 expression at the esophagogastric junction is increased in gastro-esophageal reflux disease (GERD). Peptides 25, 771–777 (2004).
McIntire, M. G., Soucy, G., Vaughan, T. L., Shahsafaei, A. & Odze, R. D. MUC2 is a highly specific marker of goblet cell metaplasia in the distal esophagus and gastro-esophageal junction. Am. J. Surg. Pathol. 35, 1007–1013 (2011).
Reis, C. A. et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 59, 1003–1007 (1999).
White, N. M. et al. Barrett's esophagus and cardiac intestinal metaplasia: two conditions within the same spectrum. Can. J. Gastroenterol. 22, 369–375 (2008).
Oh, D. S. et al. The gene expression profile of cardia intestinal metaplasia is similar to that of Barrett's esophagus, not gastric intestinal metaplasia. Dis. Esophagus 24, 516–522 (2011).
Srivastava, S. et al. Immunohistochemical analysis of metaplastic non-goblet columnar lined oesophagus shows phenotypic similarities to Barrett's oesophagus: a study in an Asian population. Dig. Liver Dis. 46, 170–175 (2014).
Rindi, G. et al. A mixed pattern of endocrine cells in metaplastic Barrett's oesophagus. Evidence that the epithelium derives from a pluripotential stem cell. Histochemistry 87, 377–383 (1987).
Otsuka, T. et al. Coexistence of gastric- and intestinal-type endocrine cells in gastric and intestinal mixed intestinal metaplasia of the human stomach. Pathol. Int. 55, 170–179 (2005).
Paull, A. et al. The histologic spectrum of Barrett's esophagus. N. Engl. J. Med. 295, 476–480 (1976).
Burke, Z. D. & Tosh, D. Barrett's metaplasia as a paradigm for understanding the development of cancer. Curr. Opin. Genet. Dev. 22, 494–499 (2012).
Filipe, M. I. & Jass, J. R. in Gastric Carcinoma (eds Filipe, M. I. & Jass, J. R.) 87–115 (Churchill Livingstone London, 1986).
Solcia, E. et al. Intestinal and diffuse gastric cancers arise in a different background of Helicobacter pylori gastritis through different gene involvement. Am. J. Surg. Pathol. 20 (Suppl. 1), S8–S22 (1996).
Tanaka, H. et al. Expression of small intestinal and colonic phenotypes in complete intestinal metaplasia of the human stomach. Virchows Arch. 447, 806–815 (2005).
Tatematsu, M., Tsukamoto, T. & Inada, K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 94, 135–141 (2003).
Tsukamoto, T., Mizoshita, T. & Tatematsu, M. Gastric-and-intestinal mixed-type intestinal metaplasia: aberrant expression of transcription factors and stem cell intestinalization. Gastric Cancer 9, 156–166 (2006).
Correa, P., Piazuelo, M. B. & Wilson, K. T. Pathology of gastric intestinal metaplasia: clinical implications. Am. J. Gastroenterol. 105, 493–498 (2010).
Gottfried, M. R., McClave, S. A. & Boyce, H. W. Incomplete intestinal metaplasia in the diagnosis of columnar-lined esophagus (Barrett's esophagus). Am. J. Clin. Pathol. 42, 741–746 (1989).
Chandrasoma, P. T. et al. Distribution and significance of epithelial types in columnar-lined esophagus. Am. J. Surg. Pathol. 25, 1188–1193 (2001).
Thompson, J. J., Zinsser, K. R. & Enterline, H. T. Barrett's metaplasia and adenocarcinoma of the esophagus and gastroesophageal junction. Hum. Pathol. 14, 42–60 (1983).
Theodorou, D. et al. Intraluminal pH and goblet cell density in Barrett's esophagus. J. Gastrointest. Surg. 16, 469–474 (2012).
Going, J. J. et al. Zoning of mucosal phenotype, dysplasia, and telomerase activity measured by telomerase repeat assay protocol in Barrett's esophagus. Neoplasia 6, 85–92 (2004).
Stairs, D. B. et al. Cdx1 and c-Myc foster the initiation of transdifferentiation of the normal esophageal squamous epithelium toward Barrett's esophagus. PLoS ONE 3, e3534 (2008).
Slack, J. M. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat. Rev. Mol. Cell Biol. 8, 369–378 (2007).
Slack, J. M. Epithelial metaplasia and the second anatomy. Lancet 2, 268–271 (1986).
Okada, T. S. Transdifferentiation: flexibility in cell differentiation (Clarendon Press, 1991).
Nomura, S., Kaminishi, M., Sugiyama, K., Oohara, T. & Esumi, H. Clonal analysis of isolated single fundic and pyloric gland of stomach using X-linked polymorphism. Biochem. Biophys. Res. Commun. 226, 385–390 (1996).
Nomura, S., Kaminishi, M., Sugiyama, K., Oohara, T. & Esumi, H. Clonal analysis of isolated intestinal metaplastic glands of stomach using X linked polymorphism. Gut 42, 663–668 (1998).
Mihara, M. et al. Methylation of multiple genes in gastric glands with intestinal metaplasia: a disorder with polyclonal origins. Am. J. Pathol. 169, 1643–1651 (2006).
Gutierrez-Gonzalez, L. et al. The clonal origins of dysplasia from intestinal metaplasia in the human stomach. Gastroenterology 140, 1251–1260 (2011).
Nicholson, A. M. et al. Barrett's metaplasia glands are clonal, contain multiple stem cells and share a common squamous progenitor. Gut 61, 1380–1389 (2011).
Niwa, T. et al. Mixed gastric- and intestinal-type metaplasia is formed by cells with dual intestinal and gastric differentiation. J. Histochem. Cytochem. 53, 75–85 (2005).
El-Zimaity, H. M., Ramchatesingh, J., Ali Saeed, M. & Graham, D. Y. Gastric intestinal metaplasia: subtypes and natural history. J. Clin. Pathol. 54, 679–683 (2001).
Greaves, L. C. et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc. Natl Acad. Sci. USA 103, 714–719 (2006).
McDonald, S. A. et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology 134, 500–510 (2008).
Milind, R. & Attwood, S. E. Natural history of Barrett's esophagus. World J. Gastroenterol. 18, 3483–3491 (2012).
Barrett, M. T. et al. Evolution of neoplastic cell lineages in Barrett oesophagus. Nat. Genet. 22, 106–109 (1999).
Wong, D. J. et al. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett's metaplastic epithelium. Cancer Res. 61, 8284–8289 (2001).
Glickman, J. N., Shahsafaei, A. & Odze, R. D. Mucin core peptide expression can help differentiate Barrett's esophagus from intestinal metaplasia of the stomach. Am. J. Surg. Pathol. 27, 1357–1365 (2003).
Montgomery, E. in Barrett's Esophagus Vol. 1 (ed. Guili, R.) 41 (John Libbey Eurotext Ltd, 2003).
Moyes, L. H. et al. Activation of Wnt signalling promotes development of dysplasia in Barrett's oesophagus. J. Pathol. 228, 99–112 (2012).
Going, J. J. et al. Aberrant expression of minichromosome maintenance proteins 2 and 5, and Ki-67 in dysplastic squamous oesophageal epithelium and Barrett's mucosa. Gut 50, 373–377 (2002).
Lavery, D. L. The stem cell organisation, and the proliferative and gene expression profile of Barrett's epithelium, replicates pyloric-type gastric glands. Gut http://dx.doi.org/10.1136/gutjnl-2013-306508.
Wright, N. A. & Alison, M. R. in The Biology of Epithelial Cell Populations Vol. 2 645–652 (Oxford University Press, 1984).
Watari, J. et al. K-ras mutations and cell kinetics in Helicobacter pylori associated gastric intestinal metaplasia: a comparison before and after eradication in patients with chronic gastritis and gastric cancer. Clin. Pathol. 60, 921–926 (2007).
Humphries, A. & Wright, N. A. Colonic crypt organization and tumorigenesis. Nat. Rev. Cancer 8, 415–424 (2008).
Novelli, M. R. et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science 272, 1187–1190 (1996).
Novelli, M. et al. X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc. Natl Acad. Sci. USA 100, 3311–3314 (2003).
Taylor, R. W. et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest. 112, 1351–1360 (2003).
Gutierrez-Gonzalez, L. et al. Analysis of the clonal architecture of the human small intestinal epithelium establishes a common stem cell for all lineages and reveals a mechanism for the fixation and spread of mutations. J. Pathol. 217, 489–496 (2009).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141, 1762–1772 (2011).
Bobryshev, Y. V. et al. Expression of the putative stem cell marker Musashi-1 in Barrett's esophagus and esophageal adenocarcinoma. Dis. Esophagus 23, 580–589 (2010).
Vega, K. J. et al. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett's esophagus and esophageal adenocarcinoma. J. Gastroenterol. Hepatol. 27, 773–780 (2012).
Barker, N. et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Schepers, A. G. et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 337, 730–735 (2012).
Merlos-Suárez, A. et al. The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8, 511–524 (2011).
Becker, L., Huang, Q. & Mashimo, H. Immunostaining of Lgr5, an intestinal stem cell marker, in normal and premalignant human gastrointestinal tissue. ScientificWorldJournal 8, 1168–1176 (2008).
Becker, L., Huang, Q. & Mashimo, H. Lgr5, an intestinal stem cell marker, is abnormally expressed in Barrett's esophagus and esophageal adenocarcinoma. Dis. Esophagus 23, 168–174 (2010).
von Rahden, B. H. et al. LgR5 expression and cancer stem cell hypothesis: clue to define the true origin of esophageal adenocarcinomas with and without Barrett's esophagus? J. Exp. Clin. Cancer Res. 30, 23 (2011).
Kemper, K. et al. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells 30, 2378–2386 (2012).
Itzkovitz, S. et al. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat. Cell Biol. 14, 106–114 (2011).
Tytgat, G. N. J. in Barrett's Esophagus Vol. 1 (ed. Guili, R.) 73–77 (John Libbey Eurotext Ltd, 2003).
Cameron, A. J. & Lomboy, C. T. Barrett's esophagus: age, prevalence, and extent of columnar epithelium. Gastroenterology 103, 1241–1245 (1992).
Parekh, D. et al. in Barrett's Esophagus Vol. 1 (ed. Guili, R.) 25 (John Libbey Eurotext Ltd, 2003).
Öberg, S. et al. The extent of Barrett's esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J. Thorac. Cardiovasc. Surg. 117, 572–580 (1999).
Nandurkar, S. & Talley, N. J. Barrett's esophagus: the long and the short of it. Am. J. Gastroenterol. 94, 30 (1999).
Benipal, P. et al. Short segment Barrett's esophagus: relationship of age with extent of intestinal metaplasia. Am. J. Gastroenterol. 96, 3084–3088 (2001).
Manabe, N. et al. Does short-segment columnar-lined esophagus elongate during a mean follow-up period of 5.7 years. Dig. Endosc. 23, 166–172 (2011).
Gatenby, P. A., Ramus, J. R., Caygill, C. P. & Watson, A. Does the length of the columnar-lined esophagus change with time? Dis. Esophagus 20, 497–503 (2007).
Winberg, H., Lindblad, M., Lagergren, J. & Dahlstrand, H. Risk factors and chemoprevention in Barrett's esophagus—an update. Scand. J. Gastroenterol. 47, 397–406 (2012).
Reid, B. J., Kostadinov, R. & Maley, C. C. New strategies in Barrett's esophagus: integrating clonal evolutionary theory with clinical management. Clin. Cancer Res. 17, 3512–3519 (2011).
Merlo, L. M., Pepper, J. W., Reid, B. J. & Maley, C. C. Cancer as an evolutionary and ecological process. Nat. Rev. Cancer 6, 924–935 (2006).
Savarino, V., Di Mario, F. & Scarpignato, C. Proton pump inhibitors in GORD. An overview of their pharmacology, efficacy and safety. Pharmacol. Res. 59, 135–153 (2009).
Maley, C. C., Reid, B. J. & Forrest, S. Cancer prevention strategies that address the evolutionary dynamics of neoplastic cells: simulating benign cell boosters and selection for chemosensitivity. Cancer Epidemiol. Biomarkers Prev. 13, 1375–1384 (2004).
Zeki, S. S., McDonald, S. A. & Graham, T. A. Field cancerization in Barrett's esophagus. Discov. Med. 12, 371–379 (2011).
Zeki, S. S. et al. Clonal selection and persistence in dysplastic Barrett's esophagus and intramucosal cancers after failed radiofrequency ablation. Am. J. Gastroenterol. 108, 1584–1592 (2013).
Eguchi, G. & Kodama, R. Transdifferentiation. Curr. Opin. Cell Biol. 5, 1023–1028 (1993).
Sarosi, G. et al. Bone marrow progenitor cells contribute to esophageal regeneration and metaplasia in a rat model of Barrett's esophagus. Dis. Esophagus 21, 43–50 (2008).
Hutchinson, L. et al. Human Barrett's adenocarcinoma of the esophagus, associated myofibroblasts, and endothelium can arise from bone marrow-derived cells after allogeneic stem cell transplant. Stem Cells Dev. 20, 11–17 (2011).
Wang, X. et al. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell 145, 1023–1035 (2011).
Chang, C. L. et al. Retinoic acid-induced glandular differentiation of the oesophagus. Gut 56, 906–917 (2007).
Souza, R. F., Krishnan, K. & Spechler, S. J. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G211–G218 (2008).
Johns, B. A. Developmental changes in the oesophageal epithelium in man. J. Anat. 86, 431–442 (1952).
Riddell, R. H. The genesis of Barrett esophagus: has a histologic transition from gastroesophageal reflux disease-damaged epithelium to columnar metaplasia ever been seen in humans? Arch. Pathol. Lab. Med. 129, 164–169 (2005).
Glickman, J. N., Chen, Y. Y. Wang, H. H., Antonioli, D. A. & Odze, R. D. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am. J. Surg. Pathol. 25, 569–578 (2001).
Boch, J. A. et al. Distribution of cytokeratin markers in Barrett's specialized columnar epithelium. Gastroenterology 112, 760–765 (1997).
Glickman, J. N., Yang, A., Shahsafaei, A. McKeon, F. & Odze, R. D. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum. Pathol. 32, 1157–1165 (2001).
Shields, H. M. et al. Prospective evaluation of multilayered epithelium in Barrett's esophagus. Am. J. Gastroenterol. 96, 3268–3273 (2001).
Upton, M. P. et al. Multilayered epithelium may be found in patients with Barrett's epithelium and dysplasia or adenocarcinoma. Dig Dis. Sci. 51, 1783–1790 (2006).
Langner, C. et al. Multilayered epithelium at the gastroesophageal junction is a marker of gastroesophageal reflux disease: data from a prospective Central European multicenter study (histoGERD trial). Virchows Arch. 464, 409–417 (2014).
Al Yassin, T. M. & Toner, P. G. Fine structure of squamous epithelium and submucosal glands of human oesophagus. J. Anat. 123, 705–721 (1977).
Hopwood, D., Coghill, G. & Sanders, D. S. Human oesophageal submucosal glands. Their detection mucin, enzyme and secretory protein content. Histochemistry 86, 107–112 (1986).
Fellous, T. G. et al. A methodological approach to tracing cell lineage in human epithelial tissues. Stem Cells 27, 1410–1420 (2009).
Leedham, S. J. et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett's oesophagus. Gut 57, 1041–1048 (2008).
Ahnen, D. J. et al. The ulceration-associated cell lineage (UACL) reiterates the Brunner's gland differentiation programme but acquires the proliferative organization of the gastric gland. J. Pathol. 173, 317–326 (1994).
Huang, Q. & Zhang, L. Histopathologic features of esophageal glands in the region of the gastroesophageal junction in Chinese patients with gastric cardiac cancer involving the esophagus. Pathol. Lab. Med. Int. 2, 33–40 (2010).
Rouse, R. V. et al. Esophageal submucosal gland duct adenoma. Am. J. Surg. Pathol. 19, 1191–1196 (1995).
Takubo, K., Esaki, Y., Watanabe, A., Umehara, M. & Sasajima, K. Adenoma accompanied by superficial squamous cell carcinoma of the esophagus. Cancer 71, 2435–2438 (1993).
Endoh, Y., Miyawaki, M., Tamura, G., Watanabe, H. & Motoyama, T. Esophageal adenocarcinoma that probably originated in the esophageal gland duct: a case report. Pathol. Int. 49, 156–159 (1999).
Berenson, M. M., Johnson, T. D., Markowitz, N. R., Buchi, K. N. & Samowitz, W. S. Restoration of squamous mucosa after ablation of Barrett's esophageal epithelium. Gastroenterology 104, 1686–1691 (1993).
Biddlestone, L. R., Barham, C. P., Wilkinson, S. P., Barr, H. & Shepherd, N. A. The histopathology of treated Barrett's esophagus: squamous reepithelialization after acid suppression and laser and photodynamic therapy. Am. J. Surg. Pathol. 22, 239–245 (1998).
Wilkinson, S. P., Biddlestone, L., Gore, S. & Shepherd, N. A. Regression of columnar-lined (Barrett's) oesophagus with omeprazole 40 mg daily: results of 5 years of continuous therapy. Aliment. Pharmacol. Ther. 13, 1205–1209 (1999).
Sampliner, R. E., Steinbronn, K., Garewal, H. S. & Riddell, R. H. Squamous mucosa overlying columnar epithelium in Barrett's esophagus in the absence of anti-reflux surgery. Am. J. Gastroenterol. 83, 510–512 (1988).
Gudas, L. J. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim. Biophys. Acta 1821, 213–221 (2012).
Paulson, T. G. et al. Neosquamous epithelium does not typically arise from Barrett's epithelium. Clin. Cancer Res. 15, 1701–1706 (2006).
Chen, X. & Yang, C. S. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis 22, 1119–1129 (2001).
Coad, R. A. et al. On the histogenesis of Barrett's oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J. Pathol. 206, 388–394 (2005).
Lörinc, E. & Oberg, S. Submucosal glands in the columnar-lined oesophagus: evidence of an association with metaplasia and neosquamous epithelium. Histopathology 61, 53–58 (2012).
Wright, N. A. Migration of the ductular elements of gut-associated glands gives clues to the histogenesis of structures associated with responses to acid hypersecretory state: the origins of “gastric metaplasia” in the duodenum of the specialized mucosa, of Barrett's esophagus and of pseudopyloric metaplasia. Yale J. Biol. Med. 69, 147–153 (1996).
Guillem, P. G. How to make a Barrett esophagus: pathophysiology of columnar metaplasia of the esophagus. Dig. Dis. Sci. 50, 415–424 (2005).
Kerkhof, M. et al. Does CDX2 expression predict Barrett's metaplasia in oesophageal columnar epithelium without goblet cells? Aliment. Pharmacol. Ther. 24, 1613–1621 (2006).
DeMeester, S. R. & DeMeester, T. R. Columnar mucosa and intestinal metaplasia of the esophagus: fifty years of controversy. Ann. Surg. 231, 303–321 (2000).
Chandrasoma, P. T., Der, R., Ma, Y., Peters, J. & Demeester, T. Histologic classification of patients based on mapping biopsies of the gastroesophageal junction. Am. J. Surg. Pathol. 27, 929–936 (2003).
Dunn, L. J., Shenfine, J. & Griffin, S. M. Columnar metaplasia in the esophageal remnant after esophagectomy: a systematic review. Dis. Esophagus http://dx.doi.org/10.1111/dote.12129.
Meyer, W., Vollmar, F. & Bär, W. Barrett-esophagus following total gastrectomy. A contribution to its pathogenesis. Endoscopy 11, 121–126 (1979).
Oberg, S., Johansson, J., Wenner, J. & Walther, B. Metaplastic columnar mucosa in the cervical esophagus after esophagectomy. Ann. Surg. 235, 338–345 (2002).
Lord, R. V. et al. Cardiac mucosa in the remnant esophagus after esophagectomy is an acquired epithelium with Barrett's-like features. Surgery 136, 633–640 (2004).
Dresner, S. M. et al. Human model of duodenogastro-oesophageal reflux in the development of Barrett's metaplasia. Br. J. Surg. 90, 1120–1128 (2003).
Lindahl, H., Rintala, R., Sariola, H. & Louhimo, I. Cervical Barrett's esophagus: a common complication of gastric tube reconstruction. J. Pediatr. Surg. 25, 446–448 (1990).
O'Riordan, J. M. et al. Factors influencing the development of Barrett's epithelium in the esophageal remnant postesophagectomy. Am. J. Gastroenterol. 99, 205–221 (2004).
Hamilton, S. R. & Yardley, J. H. Regenerative cardiac type mucosa and acquisition of Barrett mucosa after esophagogastrostomy. Gastroenterology 72, 669–675 (1977).
Castillo, D. et al. Activation of the BMP4 pathway and early expression of CDX2 characterize non-specialized columnar metaplasia in a human model of Barrett's esophagus. J. Gastrointest. Surg. 16, 227–237 (2012).
Leodolter, A. et al. Progression of specialized intestinal metaplasia at the cardia to macroscopically evident Barrett's esophagus: an entity of concern in the ProGERD study. Scand. J. Gastroenterol. 47, 1429–1435 (2012).
Galandiuk, S. et al. Field cancerization in the intestinal epithelium of patients with Crohn's ileocolitis. Gastroenterology 142, 855–864 (2012).
Chandrasoma, P. T. et al. Definition of histopathologic changes in gastroesophageal reflux disease. Am. J. Surg. Pathol. 24, 344–351 (2000).
Nishimaki, T., Watanabe, K., Suzuki, T., Hatakeyama, K. & Watanabe, H. Early esophageal adenocarcinoma arising in a short segment of Barrett's mucosa after total gastrectomy. Am. J. Gastroenterol. 91, 1856–1857 (1996).
Guo, R. J., Suh, E. R. & Lynch, J. P. The role of Cdx proteins in intestinal development and cancer. Cancer Biol. Ther. 3, 593–601 (2004).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Baker, A. M. et al. Quantification of crypt and stem cell evolution in the normal and neoplastic human colon. Cell Rep. 8, 940–947 (2014).
Mari, L. et al. A pSMAD/CDX2 complex is essential for the intestinalization of epithelial metaplasia. Cell Rep. 7, 1197–1210 (2014).
Chaves, P. et al. Recurrent columnar-lined esophageal segments—study of the phenotypic characteristics using intestinal markers. Dis. Esophagus 15, 282–286 (2002).
Chaves, P. et al. Non-goblet cell population of Barrett's esophagus: an immunohistochemical demonstration of intestinal differentiation. Hum. Pathol. 30, 1291–1295 (1999).
Dias Pereira, A. & Chaves, P. Columnar-lined oesophagus without intestinal metaplasia: results from a cohort with a mean follow-up of 7 years. Aliment. Pharmacol. Ther. 36, 282–289 (2012).
Chandrasoma, P., Wijetunge, S., Demeester, S. R., Hagen, J. & Demeester, T. R. The histologic squamo-oxyntic gap: an accurate and reproducible diagnostic marker of gastroesophageal reflux disease. Am. J. Surg. Pathol. 34, 1574–1581 (2010).
Takubo, K., Sasajima, K., Yamashita, K., Tanaka, Y. & Fujita, K. Double muscularis mucosae in Barrett's esophagus. Hum. Pathol. 22, 1158–1161 (1991).
Peitz, U. Small-bowel metaplasia arising in the remnant esophagus after esophagojejunostomy—a [corrected] prospective study in patients with a history of total gastrectomy. Am. J. Gastroenterol. 100, 2062–2070 (2005).
Carlson, C. A. et al. Decoding cell lineage from acquired mutations using arbitrary deep sequencing. Nat. Methods 9, 78–80 (2011).
Behjati, S. et al. Genome sequencing of normal cells reveals developmental lineages and mutational processes. Nature 513, 422–425 (2014).
Acknowledgements
S.A.C.M. acknowledges funding from CORE, the MRC and Barts Charity. N.A.W. acknowledges funding from Cancer Research UK. M.J. acknowledges funding from the Dutch Cancer Society. The funders had no influence on the design or content of this Perspectives.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Stained Barrett glands. (PDF 231 kb)
Supplementary Figure 2
Intestinal metaplastic glands from the human stomach and Barrett glands are clonal. (PDF 296 kb)
Supplementary Figure 3
a | A large patch of >10 Cytochrome c oxidase (CCO)-deficient Barrett oesophagus glands before laser capture microdissection. (PDF 186 kb)
Supplementary Figure 4
a | (i) A sample of Barrett metaplasia from a patient who has undergone ablative therapy. (PDF 293 kb)
Rights and permissions
About this article
Cite this article
McDonald, S., Lavery, D., Wright, N. et al. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol 12, 50–60 (2015). https://doi.org/10.1038/nrgastro.2014.181
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2014.181
This article is cited by
-
Esophageal organoids: applications and future prospects
Journal of Molecular Medicine (2023)
-
Key molecules involved in the Th17/Treg balance are associated with the pathogenesis of reflux esophagitis and Barrett’s esophagus
Esophagus (2021)
-
Antibodies to a CA 19-9 Related Antigen Complex Identify SOX9 Expressing Progenitor Cells In Human Foetal Pancreas and Pancreatic Adenocarcinoma
Scientific Reports (2019)
-
Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer
Nature Reviews Gastroenterology & Hepatology (2018)
-
Evolution of Barrett’s esophagus through space and time at single-crypt and whole-biopsy levels
Nature Communications (2018)