Key Points
-
Fetal adrenal cells are critical for the establishment of the capsular–cortical unit of the adrenal gland; these cells populate the mesenchymal capsule to become stem cells for the underlying adult (definitive) cortex
-
The adrenal capsular and subcapsular cell populations have a crucial role in gland maintenance; paracrine signals between the capsule and the cortex coordinate normal homeostasis by regulating capsular and subcapsular cellular lineages
-
Genetic defects in signalling pathways that regulate adrenocortical stem and progenitor cells contribute to diseases across the spectrum of adrenal failure and neoplasia
Abstract
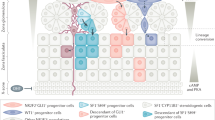
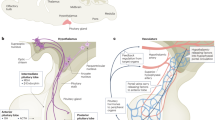
Stem cells are endowed with the potential for self-renewal and multipotency. Pluripotent embryonic stem cells have an early role in the formation of the three germ layers (ectoderm, mesoderm and endoderm), whereas adult tissue stem cells and progenitor cells are critical mediators of organ homeostasis. The adrenal cortex is an exceptionally dynamic endocrine organ that is homeostatically maintained by paracrine and endocrine signals throughout postnatal life. In the past decade, much has been learned about the stem and progenitor cells of the adrenal cortex and the multiple roles that these cell populations have in normal development and homeostasis of the adrenal gland and in adrenal diseases. In this Review, we discuss the evidence for the presence of adrenocortical stem cells, as well as the various signalling molecules and transcriptional networks that are critical for the embryological establishment and postnatal maintenance of this vital population of cells. The implications of these pathways and cells in the pathophysiology of disease are also addressed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Young, H. E. & Black, A. C. Jr. Adult stem cells. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 276, 75–102 (2004).
Alison, M. R. & Islam, S. Attributes of adult stem cells. J. Pathol. 217, 144–160 (2009).
Arnold, J. Ein Beitrag zu der feineren Struktur und dem Chemismus der Nebennieren [German]. Arch. Pathol. Anat. Physiol. Klin. Med. 35, 64–107 (1866).
Mesiano, S. & Jaffe, R. B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 18, 378–403 (1997).
Heikkilä, M. et al. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology 143, 4358–4365 (2002).
Nishimoto, K. et al. Adrenocortical zonation in humans under normal and pathological conditions. J. Clin. Endocrinol. Metab. 95, 2296–2305 (2010).
Mitani, F. et al. A novel cell layer without corticosteroid-synthesizing enzymes in rat adrenal cortex: histochemical detection and possible physiological role. Endocrinology 135, 431–438 (1994).
Bragulla, H., Hirschberg, R. M., Schlotfeldt, U., Stede, M. & Budras, K. D. On the structure of the adrenal gland of the common seal (Phoca vitulina vitulina). Anat. Histol. Embryol. 33, 263–272 (2004).
Ingle, D. & Higgins, G. Autotransplantation and regeneration of the adrenal gland. Endocrinology 22, 458–464 (1938).
Thomas, M., Northrup, S. R. & Hornsby, P. J. Adrenocortical tissue formed by transplantation of normal clones of bovine adrenocortical cells in scid mice replaces the essential functions of the animals' adrenal glands. Nat. Med. 3, 978–983 (1997).
Thomas, M. & Hornsby, P. J. Transplantation of primary bovine adrenocortical cells into scid mice. Mol. Cell. Endocrinol. 153, 125–136 (1999).
Bertholet, J. Y. Proliferative activity and cell migration in the adrenal cortex of fetal and neonatal rats: an autoradiographic study. J. Endocrinol. 87, 1–9 (1980).
Zajicek, G., Ariel, I. & Arber, N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J. Endocrinol. 111, 477–482 (1986).
McNicol, A. M. & Duffy, A. E. A study of cell migration in the adrenal cortex of the rat using bromodeoxyuridine. Cell Tissue Kinet. 20, 519–526 (1987).
Chang, S. P. et al. Cell proliferation, movement and differentiation during maintenance of the adult mouse adrenal cortex. PLoS ONE 8, e81865 (2013).
Morley, S. D. et al. Variegated expression of a mouse steroid 21-hydroxylase/β-galactosidase transgene suggests centripetal migration of adrenocortical cells. Mol. Endocrinol. 10, 585–598 (1996).
Hu, M. et al. Tissue-specific, hormonal, and developmental regulation of SCC–LacZ expression in transgenic mice leads to adrenocortical zone characterization. Endocrinology 140, 5609–5618 (1999).
Iannaccone, P., Morley, S., Skimina, T., Mullins, J. & Landini, G. Cord-like mosaic patches in the adrenal cortex are fractal: implications for growth and development. FASEB J. 17, 41–43 (2003).
Salmon, T. N. & Zwemer, R. L. A study of the life history of cortico-adrenal gland cells of the rat by means of trypan blue injections. Anat. Rec. 80, 421–429 (1941).
Uotila, U. U. The early embryological development of the fetal and permanent adrenal cortex in man. Anat. Rec. 76, 183–203 (1940).
Luo, X., Ikeda, Y. & Parker, K. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77, 481–490 (1994).
Hatano, O., Takakusu, A., Nomura, M. & Morohashi, K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1, 663–671 (1996).
Anderson, D., Carnahan, J., Michelsohn, A. & Patterson, P. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 11, 3507–3519 (1991).
Mitani, F., Mukai, K., Miyamoto, H., Suematsu, M. & Ishimura, Y. Development of functional zonation in the rat adrenal cortex. Endocrinology 140, 3342–3353 (1999).
Conley, A. J., Bernstein, R. M. & Nguyen, A. D. Adrenarche in nonhuman primates: the evidence for it and the need to redefine it. J. Endocrinol. 214, 121–131 (2012).
Schnabel, C. A., Selleri, L. & Cleary, M. L. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis 37, 123–130 (2003).
Kreidberg, J. A. et al. WT-1 is required for early kidney development. Cell 74, 679–691 (1993).
Moore, A. W., McInnes, L., Kreidberg, J., Hastie, N. D. & Schedl, A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development 126, 1845–1857 (1999).
Bamforth, S. D. et al. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 29, 469–474 (2001).
Shinoda, K. et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev. Dyn. 204, 22–29 (1995).
Ingraham, H. A. et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 8, 2302–2312 (1994).
Morohashi, K. et al. Structural and functional abnormalities in the spleen of an mFtz-F1 gene-disrupted mouse. Blood 93, 1586–1594 (1999).
Zubair, M., Ishihara, S., Oka, S., Okumura, K. & Morohashi, K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox–Pbx1–Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol. Cell. Biol. 26, 4111–4121 (2006).
Zubair, M., Parker, K. & Morohashi, K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol. Cell. Biol. 28, 7030–7040 (2008).
Briscoe, J. & Thérond, P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell. Biol. 14, 418–431 (2013).
King, P., Paul, A. & Laufer, E. Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages. Proc. Natl Acad. Sci. USA 106, 21185–21190 (2009).
Ching, S. & Vilain, E. Targeted disruption of Sonic Hedgehog in the mouse adrenal leads to adrenocortical hypoplasia. Genesis 47, 628–637 (2009).
Huang, C. C., Miyagawa, S., Matsumaru, D., Parker, K. L. & Yao, H. H. Progenitor cell expansion and organ size of mouse adrenal is regulated by sonic hedgehog. Endocrinology 151, 1119–1128 (2010).
Wood, M. A. et al. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development 140, 4522–4532 (2013).
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell. Biol. 10, 207–217 (2009).
Bandiera, R. et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev. Cell 27, 5–18 (2013).
Wood, M. A. & Hammer, G. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol. Cell. Endocrinol. 336, 206–212 (2011).
Gottschau, M. Struktur und embryonale Entwicklung der Nebennieren bei Säugetieren. Archiv fur Anatomie und Physiologie, 412–458 (1883).
Deane, H. W. & Greep, R. O. A morphological and histochemical study of the rat's adrenal cortex after hypophysectomy, with comments on the liver. Am. J. Anat. 79, 117–45 (1946).
Freedman, B. D. et al. Adrenocortical zonation results from lineage conversion of differentiated zona glomerulosa cells. Dev. Cell 26, 666–673 (2013).
Lala, D. S., Rice, D. A. & Parker, K. L. Steroidogenic factor 1, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol. Endocrinol. 6, 1249–1258 (1992).
Honda, S. et al. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J. Biol. Chem. 268, 7494–502 (1993).
Ikeda, Y., Shen, W., Ingraham, H. & Parker, K. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 8, 654–662 (1994).
Bland, M. et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc. Natl Acad. Sci. USA 97, 14488–14493 (2000).
Beuschlein, F. et al. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology 143, 3122–3135 (2002).
Achermann, J. C., Ito, M., Ito, M., Hindmarsh, P. C. & Jameson, J. L. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat. Genet. 22, 125–126 (1999).
Achermann, J. C. et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J. Clin. Endocrinol. Metab. 87, 1829–1833 (2002).
Zubair, M., Oka, S., Parker, K. & Morohashi, K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol. Endocrinol. 23, 1657–1667 (2009).
Crawford, P., Sadovsky, Y. & Milbrandt, J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol. Cell. Biol. 17, 3997–4006 (1997).
Gondo, S. et al. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells 9, 1239–1247 (2004).
Doghman, M. et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol. Endocrinol. 21, 2968–2987 (2007).
Figueiredo, B. et al. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J. Clin. Endocrinol. Metab. 90, 615–619 (2005).
Pianovski, M. et al. SF-1 overexpression in childhood adrenocortical tumours. Eur. J. Cancer 42, 1040–1043 (2006).
Almeida, M. Q. et al. Steroidogenic factor 1 overexpression and gene amplification are more frequent in adrenocortical tumors from children than from adults. J. Clin. Endocrinol. Metab. 95, 1458–1462 (2010).
Sbiera, S. et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J. Clin. Endocrinol. Metab. 95, E161–E171 (2010).
Kim, A. et al. In search of adrenocortical stem and progenitor cells. Endocr. Rev. 30, 241–263 (2009).
Hoivik, E. A., Lewis, A. l., Aumo, L. & Bakke, M. Molecular aspects of steroidogenic factor 1 (SF-1). Mol. Cell. Endocrinol. 315, 27–39 (2010).
Zanaria, E. et al. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature 372, 635–641 (1994).
Ito, M., Yu, R. & Jameson, J. L. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol. Cell. Biol. 17, 1476–1483 (1997).
Niakan, K. K. & McCabe, E. R. DAX1 origin, function, and novel role. Mol. Genet. Metab. 86, 70–83 (2005).
Crawford, P. A., Dorn, C., Sadovsky, Y. & Milbrandt, J. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol. Cell. Biol. 18, 2949–2956 (1998).
Altincicek, B. et al. Interaction of the corepressor Alien with DAX-1 is abrogated by mutations of DAX-1 involved in adrenal hypoplasia congenita. J. Biol. Chem. 275, 7662–7667 (2000).
Xu, B. et al. Dax-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidogenic factor 1 in steroidogenesis. Mol. Cell. Biol. 29, 1719–1734 (2009).
Babu, P. et al. Interaction between Dax-1 and steroidogenic factor-1 in vivo: increased adrenal responsiveness to ACTH in the absence of Dax-1. Endocrinology 143, 665–673 (2002).
Zazopoulos, E., Lalli, E., Stocco, D. M. & Sassone-Corsi, P. DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature 390, 311–315 (1997).
Niakan, K. K. et al. Novel role for the orphan nuclear receptor Dax1 in embryogenesis, different from steroidogenesis. Mol. Genet. Metab. 88, 261–271 (2006).
Khalfallah, O., Rouleau, M., Barbry, P., Bardoni, B. & Lalli, E. Dax-1 knockdown in mouse embryonic stem cells induces loss of pluripotency and multilineage differentiation. Stem Cells 27, 1529–1537 (2009).
Kelly, V., Xu, B., Kuick, R., Koenig, R. & Hammer, G. Dax1 up-regulates Oct4 expression in mouse embryonic stem cells via LRH-1 and SRA. Mol. Endocrinol. 24, 2281–2291 (2010).
Kelly, V. R. & Hammer, G. D. LRH-1 and Nanog regulate Dax1 transcription in mouse embryonic stem cells. Mol. Cell. Endocrinol. 332, 116–124 (2011).
Scheys, J., Heaton, J. & Hammer, G. Evidence of adrenal failure in aging Dax1-deficient mice. Endocrinology 152, 3430–3439 (2011).
Muscatelli, F. et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372, 672–676 (1994).
Peter, M., Viemann, M., Partsch, C. J. & Sippell, W. G. Congenital adrenal hypoplasia: clinical spectrum, experience with hormonal diagnosis, and report on new point mutations of the DAX-1 gene. J. Clin. Endocrinol. Metab. 83, 2666–2674 (1998).
Landau, Z. et al. Clinical and genetic heterogeneity of congenital adrenal hypoplasia due to NR0B1 gene mutations. Clin. Endocrinol. 72, 448–454 (2010).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Nusse, R. Wnt signaling. Cold Spring Harb. Perspect. Biol. 4, a011163 (2012).
Kim, A. et al. Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135, 2593–2602 (2008).
Mizusaki, H. et al. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by Wnt4 in the female developing gonad. Mol. Endocrinol. 17, 507–519 (2003).
Berthon, A. et al. Constitutive β-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development. Hum. Mol. Genet. 19, 1561–1576 (2010).
Heaton, J. et al. Progression to adrenocortical tumorigenesis in mice and humans through insulin-like growth factor 2 and β-catenin. Am. J. Pathol. 181, 1017–1033 (2012).
Berthon, A. et al. WNT/β-catenin signalling is activated in aldosterone producing adenomas and controls aldosterone production. Hum. Mol. Genet. 23, 889–905 (2013).
Walczak, E. M. et al. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol. Endocrinol. 28, 1471–1486 (2014).
Gaujoux, S. et al. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin. Cancer Res. 16, 5133–5141 (2010).
Tissier, F. et al. Mutations of β-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 65, 7622–7627 (2005).
Assie, G. et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 46, 607–612 (2014).
Park, J. S., Valerius, M. T. & McMahon, A. P. Wnt/β-catenin signaling regulates nephron induction during mouse kidney development. Development 134, 2533–2539 (2007).
Boyer, A. et al. CTNNB1 signaling in Sertoli cells downregulates spermatogonial stem cell activity via WNT4. PLoS ONE 7, e29764 (2012).
Jeays-Ward, K. et al. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130, 3663–3670 (2003).
Mandel, H. et al. SERKAL syndrome: an autosomal-recessive disorder caused by a loss-of-function mutation in WNT4. Am. J. Hum. Genet. 82, 39–47 (2008).
Kang, S., Graham, J. M., Olney, A. H. & Biesecker, L. G. GLI3 frameshift mutations cause autosomal dominant Pallister–Hall syndrome. Nat. Genet. 15, 266–268 (1997).
Hall, J. G. et al. Congenital hypothalamic hamartoblastoma, hypopituitarism, imperforate anus and postaxial polydactyly—a new syndrome? Part I: clinical, causal, and pathogenetic considerations. Am. J. Med. Genet. 7, 47–74 (1980).
Böse, J., Grotewold, L. & Rüther, U. Pallister–Hall syndrome phenotype in mice mutant for Gli3. Hum. Mol. Genet. 11, 1129–1135 (2002).
Laufer, E., Kesper, D., Vortkamp, A. & King, P. Sonic hedgehog signaling during adrenal development. Mol. Cell. Endocrinol. 351, 19–27 (2012).
Lanner, F. & Rossant, J. The role of FGF/Erk signaling in pluripotent cells. Development 137, 3351–3360 (2010).
Guasti, L., Candy Sze, W. C., McKay, T., Grose, R. & King, P. J. FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development. Mol. Cell. Endocrinol. 371, 182–188 (2013).
Ornitz, D. M. et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 271, 15292–15297 (1996).
Kim, Y. et al. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc. Natl Acad. Sci. USA 104, 16558–16563 (2007).
Revest, J. M. et al. Fibroblast growth factor receptor 2-IIIb acts upstream of Shh and Fgf4 and is required for limb bud maintenance but not for the induction of Fgf8, Fgf10, Msx1, or Bmp4. Dev. Biol. 231, 47–62 (2001).
Hajihosseini, M. K., Wilson, S., De Moerlooze, L. & Dickson, C. A splicing switch and gain-of-function mutation in FgfR2-IIIc hemizygotes causes Apert/Pfeiffer-syndrome-like phenotypes. Proc. Natl Acad. Sci. USA 98, 3855–3860 (2001).
Gospodarowicz, D. & Handley, H. H. Stimulation of division of Y1 adrenal cells by a growth factor isolated from bovine pituitary glands. Endocrinology 97, 102–107 (1975).
Gospodarowicz, D., Ill, C. R., Hornsby, P. J. & Gill, G. N. Control of bovine adrenal cortical cell proliferation by fibroblast growth factor. Lack of effect of epidermal growth factor. Endocrinology 100, 1080–1089 (1977).
Basile, D. P. & Holzwarth, M. A. Basic fibroblast growth factor may mediate proliferation in the compensatory adrenal growth response. Am. J. Physiol. 265, R1253–R1261 (1993).
Basile, D. P. & Holzwarth, M. A. Basic fibroblast growth factor receptor in the rat adrenal cortex: effects of suramin and unilateral adrenalectomy on receptor numbers. Endocrinology 134, 2482–2489 (1994).
de Fraipont, F. et al. Gene expression profiling of human adrenocortical tumors using complementary deoxyribonucleic acid microarrays identifies several candidate genes as markers of malignancy. J. Clin. Endocrinol. Metab. 90, 1819–1829 (2005).
West, A. N. et al. Gene expression profiling of childhood adrenocortical tumors. Cancer Res. 67, 600–608 (2007).
Brito, L. P. et al. The role of fibroblast growth factor receptor 4 overexpression and gene amplification as prognostic markers in pediatric and adult adrenocortical tumors. Endocr. Relat. Cancer 19, L11–L13 (2012).
Watabe, T. & Miyazono, K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 19, 103–115 (2009).
Spencer, S. J., Rabinovici, J., Mesiano, S., Goldsmith, P. C. & Jaffe, R. B. Activin and inhibin in the human adrenal gland. Regulation and differential effects in fetal and adult cells. J. Clin. Invest. 90, 142–149 (1992).
Suzuki, J. et al. Novel action of activin and bone morphogenetic protein in regulating aldosterone production by human adrenocortical cells. Endocrinology 145, 639–649 (2004).
Hofland, J. & de Jong, F. H. Inhibins and activins: their roles in the adrenal gland and the development of adrenocortical tumors. Mol. Cell. Endocrinol. 359, 92–100 (2012).
Matzuk, M. M. et al. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc. Natl Acad. Sci. USA 91, 8817–8821 (1994).
Beuschlein, F. et al. Activin induces x-zone apoptosis that inhibits luteinizing hormone-dependent adrenocortical tumor formation in inhibin-deficient mice. Mol. Cell. Biol. 23, 3951–3964 (2003).
Looyenga, B. & Hammer, G. Origin and identity of adrenocortical tumors in inhibin knockout mice: implications for cellular plasticity in the adrenal cortex. Mol. Endocrinol. 20, 2848–2863 (2006).
Alevizaki, M., Saltiki, K., Mantzou, E., Anastasiou, E. & Huhtaniemi, I. The adrenal gland may be a target of LH action in postmenopausal women. Eur. J. Endocrinol. 154, 875–881 (2006).
Fottner, C., Niederle, I. M. & Weber, M. J. in Adrenocortical Carcinoma (eds Hammer, G. & Else, T.) 235–262 (Springer, 2011).
Mesiano, S., Mellon, S. H. & Jaffe, R. B. Mitogenic action, regulation, and localization of insulin-like growth factors in the human fetal adrenal gland. J. Clin. Endocrinol. Metab. 76, 968–976 (1993).
Penhoat, A., Chatelain, P. G., Jaillard, C. & Saez, J. M. Characterization of insulin-like growth factor I and insulin receptors on cultured bovine adrenal fasciculata cells. Role of these peptides on adrenal cell function. Endocrinology 122, 2518–2526 (1988).
Belgorosky, A., Baquedano, M. S., Guercio, G. & Rivarola, M. A. Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: implications for the development of adrenarche. Rev. Endocr. Metab. Disord. 10, 51–61 (2009).
Pitetti, J. L. et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 9, e1003160 (2013).
Penhoat, A., Rainey, W. E., Viard, I. & Saez, J. M. Regulation of adrenal cell-differentiated functions by growth factors. Horm. Res. 42, 39–43 (1994).
Weksberg, R., Shuman, C. & Smith, A. C. Beckwith–Wiedemann syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 137C, 12–23 (2005).
Beckwith, J. Extreme cytomegaly of the adrenal fetal cortex, omphalocele, hyperplasia of kidneys and pancreas, and Leydig-cell hyperplasia: another syndrome? Presented at the 11th Annual Meeting of the Western Society for Pediatric Research, Los Angeles, USA (1963).
Lapunzina, P. Risk of tumorigenesis in overgrowth syndromes: a comprehensive review. Am. J. Med. Genet. C Semin. Med. Genet. 137C, 53–71 (2005).
Giordano, T. J. et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 15, 668–676 (2009).
de Reyniès, A. et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 27, 1108–1115 (2009).
Assié, G., Jouinot, A. & Bertherat, J. The 'omics' of adrenocortical tumours for personalized medicine. Nat. Rev. Endocrinol. 10, 215–228 (2014).
Drelon, C. et al. Analysis of the role of Igf2 in adrenal tumour development in transgenic mouse models. PLoS ONE 7, e44171 (2012).
Arboleda, V. A. et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat. Genet. 44, 788–792 (2012).
Hamajima, N., Johmura, Y., Suzuki, S., Nakanishi, M. & Saitoh, S. Increased protein stability of CDKN1C causes a gain-of-function phenotype in patients with IMAGe syndrome. PLoS ONE 8, e75137 (2013).
Hattangady, N., Olala, L., Bollag, W. & Rainey, W. Acute and chronic regulation of aldosterone production. Mol. Cell. Endocrinol. 350, 151–162 (2012).
Miller, W. & Auchus, R. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Oliverio, M. I. et al. Reduced growth, abnormal kidney structure, and type 2 (AT2) angiotensin receptor-mediated blood pressure regulation in mice lacking both AT1A and AT1B receptors for angiotensin II. Proc. Natl Acad. Sci. USA 95, 15496–15501 (1998).
Chida, D. et al. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. Proc. Natl Acad. Sci. USA 104, 18205–18210 (2007).
Karpac, J. et al. Development, maintenance, and function of the adrenal gland in early postnatal proopiomelanocortin-null mutant mice. Endocrinology 146, 2555–2562 (2005).
Dallman, M. F. Control of adrenocortical growth in vivo. Endocr. Res. 10, 213–242 (1984).
Mitani, F., Mukai, K., Miyamoto, H., Suematsu, M. & Ishimura, Y. The undifferentiated cell zone is a stem cell zone in adult rat adrenal cortex. Biochim. Biophys. Acta 1619, 317–324 (2003).
Nishimoto, K., Harris, R. B., Rainey, W. E. & Seki, T. Sodium deficiency regulates rat adrenal zona glomerulosa gene expression. Endocrinology 155, 1363–1372 (2014).
Ferreira, J. G., Cruz, C. D., Neves, D. & Pignatelli, D. Increased extracellular signal regulated kinases phosphorylation in the adrenal gland in response to chronic ACTH treatment. J. Endocrinol. 192, 647–658 (2007).
Yates, R. et al. Adrenocortical development, maintenance, and disease. Curr. Top. Dev. Biol. 106, 239–312 (2013).
Voutilainen, R. & Miller, W. L. Coordinate tropic hormone regulation of mRNAs for insulin-like growth factor II and the cholesterol side-chain-cleavage enzyme, P450scc [corrected], in human steroidogenic tissues. Proc. Natl Acad. Sci. USA 84, 1590–1594 (1987).
Mesiano, S., Mellon, S. H., Gospodarowicz, D., Di Blasio, A. M. & Jaffe, R. B. Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: a model for adrenal growth regulation. Proc. Natl Acad. Sci. USA 88, 5428–5432 (1991).
Gummow, B., Scheys, J., Cancelli, V. & Hammer, G. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol. Endocrinol. 20, 2711–2723 (2006).
Almeida, M. Q. et al. Activation of cyclic AMP signaling leads to different pathway alterations in lesions of the adrenal cortex caused by germline PRKAR1A defects versus those due to somatic GNAS mutations. J. Clin. Endocrinol. Metab. 97, E687–E693 (2012).
Sidhu, A., Debelenko, L. & Misra, V. K. Infantile adrenocortical tumor with an activating GNAS1 mutation. J. Clin. Endocrinol. Metab. 98, E115–E118 (2013).
Goh, G. et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat. Genet. 46, 613–617 (2014).
Fragoso, M. C. et al. Cushing's syndrome secondary to adrenocorticotropin-independent macronodular adrenocortical hyperplasia due to activating mutations of GNAS1 gene. J. Clin. Endocrinol. Metab. 88, 2147–2151 (2003).
Weinstein, L. S. et al. Activating mutations of the stimulatory G protein in the McCune–Albright syndrome. N. Engl. J. Med. 325, 1688–1695 (1991).
Beuschlein, F. et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing's syndrome. N. Engl. J. Med. 370, 1019–1028 (2014).
Cao, Y. et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing's syndrome. Science 344, 913–917 (2014).
Sato, Y. et al. Recurrent somatic mutations underlie corticotropin-independent Cushing's syndrome. Science 344, 917–920 (2014).
Groussin, L. et al. Mutations of the PRKAR1A gene in Cushing's syndrome due to sporadic primary pigmented nodular adrenocortical disease. J. Clin. Endocrinol. Metab. 87, 4324–4329 (2002).
Kirschner, L. S. et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat. Genet. 26, 89–92 (2000).
Griffin, K. J. et al. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res. 64, 8811–8815 (2004).
Griffin, K. J. et al. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J. Med. Genet. 41, 923–931 (2004).
Almeida, M. Q. et al. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum. Mol. Genet. 19, 1387–1398 (2010).
Sahut-Barnola, I. et al. Cushing's syndrome and fetal features resurgence in adrenal cortex-specific Prkar1a knockout mice. PLoS Genet. 6, e1000980 (2010).
Horvath, A. et al. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat. Genet. 38, 794–800 (2006).
Horvath, A. et al. A cAMP-specific phosphodiesterase (PDE8B) that is mutated in adrenal hyperplasia is expressed widely in human and mouse tissues: a novel PDE8B isoform in human adrenal cortex. Eur. J. Hum. Genet. 16, 1245–1253 (2008).
Vezzosi, D. et al. Phosphodiesterase 11A (PDE11A) gene defects in patients with ACTH-independent macronodular adrenal hyperplasia (AIMAH): functional variants may contribute to genetic susceptibility of bilateral adrenal tumors. J. Clin. Endocrinol. Metab. 97, E2063–E2069 (2012).
Libe, R. et al. Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors. Clin. Cancer Res. 14, 4016–4024 (2008).
Tullio-Pelet, A. et al. Mutant WD-repeat protein in triple-A syndrome. Nat. Genet. 26, 332–335 (2000).
Beamer, W. G. et al. Adrenocortical dysplasia: a mouse model system for adrenocortical insufficiency. J. Endocrinol. 141, 33–43 (1994).
Kume, T., Deng, K. & Hogan, B. L. Minimal phenotype of mice homozygous for a null mutation in the forkhead/winged helix gene, Mf2. Mol. Cell. Biol. 20, 1419–1425 (2000).
Assié, G. et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N. Engl. J. Med. 369, 2105–2114 (2013).
Beuschlein, F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 45, 440 (2013).
Scholl, U. I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 45, 1050–1054 (2013).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Skogseid, B. et al. Clinical and genetic features of adrenocortical lesions in multiple endocrine neoplasia type 1. J. Clin. Endocrinol. Metab. 75, 76–81 (1992).
Kjellman, M. et al. Genotyping of adrenocortical tumors: very frequent deletions of the MEN1 locus in 11q13 and of a 1-centimorgan region in 2p16. J. Clin. Endocrinol. Metab. 84, 730–735 (1999).
Karamurzin, Y. et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum. Pathol. 43, 1677–1687 (2012).
Ragazzon, B. et al. Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas. Eur. J. Endocrinol. 170, 385–391 (2014).
Li, F. P. et al. A cancer family syndrome in twenty-four kindreds. Cancer Res. 48, 5358–5362 (1988).
Hisada, M., Garber, J. E., Fung, C. Y., Fraumeni, J. F. Jr & Li, F. P. Multiple primary cancers in families with Li–Fraumeni syndrome. J. Natl Cancer Inst. 90, 606–611 (1998).
Bougeard, G. et al. Molecular basis of the Li–Fraumeni syndrome: an update from the French LFS families. J. Med. Genet. 45, 535–538 (2008).
Acknowledgements
We thank J. Heaton (University of Michigan, Ann Arbor, MI, USA) for helpful discussions and critical reading of the manuscript.
Author information
Authors and Affiliations
Contributions
E.M.W. researched the data for the article and wrote the article. E.M.W. and G.D.H. contributed equally to discussion of the content of the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Walczak, E., Hammer, G. Regulation of the adrenocortical stem cell niche: implications for disease. Nat Rev Endocrinol 11, 14–28 (2015). https://doi.org/10.1038/nrendo.2014.166
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.166
This article is cited by
-
A pan-tissue survey of mosaic chromosomal alterations in 948 individuals
Nature Genetics (2023)
-
FGF/FGFR signaling in adrenocortical development and tumorigenesis: novel potential therapeutic targets in adrenocortical carcinoma
Endocrine (2022)
-
Stem/progenitor cells in fetuses and newborns: overview of immunohistochemical markers
Cell Regeneration (2021)
-
Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence
Nature (2021)
-
β-Catenin and FGFR2 regulate postnatal rosette-based adrenocortical morphogenesis
Nature Communications (2020)