Abstract
Mature outcomes from adjuvant endocrine therapy trials in estrogen receptor-positive breast cancer have enabled comparisons with neoadjuvant clinical trials that have parallel randomizations of treatment in terms of the response of disseminated disease versus the local response within the breast. Imprecise end points, such as 'clinical response', have produced inconsistent results regarding the relationship between neoadjuvant and adjuvant endocrine therapy outcomes. However, the proliferation marker Ki-67, measured during neoadjuvant treatment, has predicted accurately and consistently the results of much larger studies in the adjuvant setting. In this Review, we summarize these trials and discuss the implications for the design of future adjuvant endocrine therapy trials. We conclude that there is sufficient evidence supporting the view that the degree of Ki-67 suppression is a reliable short-term surrogate for the adjuvant potential of endocrine drugs, at least in postmenopausal women. We propose that adjuvant endocrine therapy trials should only be conducted once adequately-powered neoadjuvant studies have indicated superior Ki-67 suppression in patients receiving experimental endocrine treatment versus the standard treatment.
Key Points
-
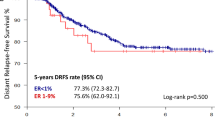
In postmenopausal women, on-treatment Ki-67 levels are more predictive of long-term outcome than baseline levels; studies validating the clinical value of this assessment as a prognostic tool are underway
-
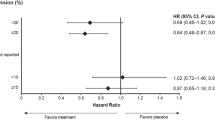
Ki-67 values in three small neoadjuvant trials predicted outcomes of large adjuvant trials; future adjuvant designs should be based on a biological superiority hypothesis generated by a neoadjuvant study
-
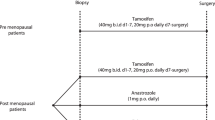
The use of on-treatment Ki-67 and the preoperative endocrine prognostic index (PEPI) in premenopausal women with estrogen receptor-positive breast cancer requires further investigation
-
High Ki-67 values can identify endocrine therapy-resistant tumors as early as 2 weeks after neoadjuvant endocrine therapy; ongoing trials are investigating the utility of this observation
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Anderson, W. F., Chen, B. E., Jatoi, I. & Rosenberg, P. S. Effects of estrogen receptor expression and histopathology on annual hazard rates of death from breast cancer. Breast Cancer Res. Treat. 100, 121–126 (2006).
Howell, A. et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet 365, 60–62 (2005).
Mouridsen, H. et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N. Engl. J. Med. 361, 766–776 (2009).
Coombes, R. C. et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N. Engl. J. Med. 350, 1081–1092 (2004).
Dowsett, M. et al. Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer—a study from the IMPACT trialists. J. Clin. Oncol. 23, 2477–2492 (2005).
Gerdes, J., Schwab, U., Lemke, H. & Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31, 13–20 (1983).
Harris, L. et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 25, 5287–5312 (2007).
Goldhirsch, A. et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 22, 1736–1747 (2011).
Cheang, M. C. et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl Cancer Inst. 101, 736–750 (2009).
Dowsett, M. et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl Cancer Inst. 103, 1656–1664 (2011).
Colozza, M., Sidoni, A. & Piccart-Gebhart, M. Value of Ki67 in breast cancer: the debate is still open. Lancet Oncol. 11, 414–415 (2010).
Luporsi, E. et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res. Treat. doi:10.1007/s10549-011-1837-z.
Ellis, M. J. et al. Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res. 63, 6523–6531 (2003).
Dowsett, M. et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin. Cancer Res. 11, 951s–958s (2005).
Dowsett, M. et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J. Natl Cancer Inst. 99, 167–170 (2007).
Thürlimann, B. et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 353, 2747–2757 (2005).
Regan, M. M. et al. Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol. 12, 1101–1108 (2011).
Eiermann, W. et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann. Oncol. 12, 1527–1532 (2001).
Ellis, M. J. et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J. Clin. Oncol. 19, 3808–3816 (2001).
Chin, S. F. et al. Using array-comparative genomic hybridization to define molecular portraits of primary breast cancers. Oncogene 26, 1959–1970 (2007).
Horobin, J. M., Preece, P. E., Dewar, J. A., Wood, R. A. & Cuschieri, A. Long-term follow-up of elderly patients with locoregional breast cancer treated with tamoxifen only. Br. J. Surg. 78, 213–217 (1991).
Ellis, M. J. et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl Cancer Inst. 100, 1380–1388 (2008).
Dowsett, M. et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl Cancer Inst. 103, 1656–1664 (2011).
Baum, M. et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359, 2131–2139 (2002).
Cuzick, J. et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol. 11, 1135–1141 (2010).
Smith, I. E. et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J. Clin. Oncol. 23, 5108–5116 (2005).
Dowsett, M. et al. Endocrine therapy, new biologicals, and new study designs for presurgical studies in breast cancer. J. Natl Cancer Inst. Monogr. 2011, 120–123 (2011).
Goss, P. E. et al. Final analysis of NCIC CTG MA.27: a randomized phase III trial of exemestane versus anastrozole in postmenopausal women with hormone receptor positive primary breast cancer [abstract]. In 33rd Annual San Antonio Breast Cancer Symposium. S1–1 (San Antonio, Texas, 2010).
Ellis, M. J. et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 29, 2342–2349 (2011).
US National Library of Medicine. ClinicalTrials.gov [online].
Dixon, J. M. et al. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J. Clin. Oncol. 26, 1671–1676 (2008).
Gnant, M. et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 12, 631–641 (2011).
Masuda, N. et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. doi:10.1016/S1470-2045(11)70373-4
Kinoshita, T. et al. Neoadjuvant anastrozole or tamoxifen for premenopausal breast cancer: Ki67 expression data from the STAGE study. J. Clin. Oncol. 29 (Suppl. 15), 501 (2011).
Baselga, J. et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 27, 2630–2637 (2009).
Baselga, J. et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. doi: 10.1056/NEJMoa1109653 (2011).
Crowder, R. J. et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 69, 3955–3962 (2009).
Sanchez, C. G. et al. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 13, R21 (2011).
Acknowledgements
This work was supported by R01 CA095614 awarded to M. J. Ellis, a 2011 AVON Foundation research grant to support R. Goncalves and CALGB Clinical Scholar Award to C. Ma. The ACOSOG Z1031 trial was supported by grants by the National Cancer Institute to the American College of Physicians Oncology Group (U24 CA114736 and U10 076,001).
Author information
Authors and Affiliations
Contributions
J. Luo researched the data for the article and reviewed the manuscript before submission. All the other authors researched the data for the manuscript, made a substantial contribution to discussion of the content, wrote, and edited and revised the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. J. Ellis declares he is consultant for Astra Zeneca, Novartis and Pfizer. He also receives research support from Astra Zeneca and he is stockholder of Bioclassifier LLC, which has licensed the PAM50 assay. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Goncalves, R., Ma, C., Luo, J. et al. Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat Rev Clin Oncol 9, 223–229 (2012). https://doi.org/10.1038/nrclinonc.2012.21
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2012.21
This article is cited by
-
Real-world data on neoadjuvant endocrine therapy in ER-positive/HER2-negative breast cancer
Breast Cancer Research and Treatment (2021)
-
The low expression of miR-1976 in plasma samples indicating its biological functions in the progression of breast cancer
Clinical and Translational Oncology (2020)
-
Current Status of Neoadjuvant Endocrine Therapy in Early Stage Breast Cancer
Current Treatment Options in Oncology (2018)
-
Neoadjuvant Therapy for Breast Cancer: State of the Science and Future Directions
Current Breast Cancer Reports (2017)
-
Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness–a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer
BMC Cancer (2013)