Abstract
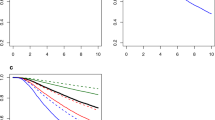
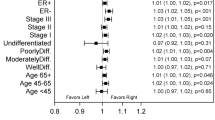
The size of a breast cancer at diagnosis has conventionally been thought of as a fundamental and critical determinant of clinical outcome. However, the tendency of some subtypes of breast cancer to behave aggressively, despite being small (≤1 cm in diameter), questions the premise that cancer size should always be considered in treatment decisions. Although there is an association between tumor size and lymph-node involvement for most tumor types, this pattern is not invariable. We speculate that the uncoupling of tumor size, lymph-node status and prognosis in some subtypes of breast cancers might reflect an underlying disproportionate relationship between the number of cancer cells with metastatic potential and the size of the cancer. Alternatively, some small cancers might harbor cells that are inherently aggressive and are likely to metastasize. These observations have implications for the screening and treatment of breast cancers, particularly for women with basal-like and BRCA1-related breast cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sotiriou, C. & Piccart, M. J. Taking gene-expression profiling to the clinic: when will molecular signatures become relevant to patient care? Nat. Rev. Cancer 7, 545–553 (2007).
Weigelt, B., Baehner, F. L. & Reis-Filho, J. S. The contribution of gene expression profiling to breast cancer classification, prognostication and prediction: a retrospective of the last decade. J. Pathol. 220, 263–280 (2010).
Mook, S. et al. Calibration and discriminatory accuracy of prognosis calculation for breast cancer with the online Adjuvant! program: a hospital-based retrospective cohort study. Lancet Oncol. 10, 1070–1076 (2009).
Singletary, S. E. et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J. Clin. Oncol. 20, 3628–3636 (2002).
Fisher, B., Slack, N. H. & Bross, I. D. Cancer of the breast: size of neoplasm and prognosis. Cancer 24, 1071–1080 (1969).
Smart, C. R., Myers, M. H. & Gloeckler, L. A. Implications from SEER data on breast cancer management. Cancer 41, 787–789 (1978).
Carter, C. L., Allen, C. & Henson, D. E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63, 181–187 (1989).
Olivotto, I. A. et al. Prediction of axillary lymph node involvement of women with invasive breast carcinoma: a multivariate analysis. Cancer 83, 948–955 (1998).
Sutcliffe, P. et al. Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review. Health Technol. Assess. 13, 1–242 (2009).
Cheang, M. C. et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J. Natl Cancer Inst. 101, 736–750 (2009).
Norton, L. & Massagué, J. Is cancer a disease of self-seeding? Nat. Med. 12, 875–878 (2006).
Norton, L. Gompertzian model of human breast cancer growth. Cancer Res. 48, 7067–7071 (1988).
Honrado, E., Benítez, J. & Palacios, J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutic implications. Mod. Pathol. 18, 1305–1320 (2005).
Bernards, R. & Weinberg, R. A. A progression puzzle. Nature 418, 823 (2002).
Collett, K. et al. A basal epithelial phenotype is more frequent in interval breast cancers compared with screen detected tumors. Cancer Epidemiol. Biomarkers Prev. 14, 1108–1112 (2005).
Komenaka, I. K. et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer 100, 2079–2083 (2004).
Rakha, E. A., Reis-Filho, J. S. & Ellis, I. O. Basal-like breast cancer: a critical review. J. Clin. Oncol. 26, 2568–2581 (2008).
Wirapati, P. et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 10, R65 (2008).
Desmedt, C. et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin. Cancer Res. 14, 5158–5165 (2008).
Van't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Sotiriou, C. & Pusztai, L. Gene-expression signatures in breast cancer. N. Engl. J. Med. 360, 790–800 (2009).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434 (2007).
Tischkowitz, M. et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer 7, 134 (2007).
Cheang, M. C. et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin. Cancer Res. 14, 1368–1376 (2008).
Foulkes, W. D. et al. The prognostic implication of the basal-like (cyclin E high/p27 low/p53+/glomeruloid-microvascular-proliferation+) phenotype of BRCA1-related breast cancer. Cancer Res. 64, 830–835 (2004).
Arnes, J. B. et al. Placental cadherin and the basal epithelial phenotype of BRCA1-related breast cancer. Clin. Cancer Res. 11, 4003–4011 (2005).
Foulkes, W. D., Grainge, M. J., Rakha, E. A., Green, A. R. & Ellis, I. O. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res. Treat. 117, 199–204 (2008).
Dent, R. et al. Time to disease recurrence in basal-type breast cancers: effects of tumor size and lymph node status. Cancer 115, 4917–4923 (2009).
Foulkes, W. D. et al. Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer 98, 1569–1577 (2003).
Rennert, G. et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N. Engl. J. Med. 357, 115–123 (2007).
Gonzalez-Angulo, A. M. et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J. Clin. Oncol. 27, 5700–5706 (2009).
Curigliano, G. et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J. Clin. Oncol. 27, 5693–5699 (2009).
Haupt, H. M., Rosen, P. P. & Kinne, D. W. Breast carcinoma presenting with axillary lymph node metastases. An analysis of specific histopathologic features. Am. J. Surg. Pathol. 9, 165–175 (1985).
Wicha, M. S., Liu, S. & Dontu, G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 66, 1883–1890 (2006).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells in solid tumors: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 (2008).
Shackleton, M., Quintana, E., Fearon, E. R. & Morrison, S. J. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 138, 822–829 (2009).
Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 1 33, 704–715 (2008).
Honeth, G. et al. The CD44+/CD24- phenotype is enriched in basal-like tumors. Breast Cancer Res. 10, R53 (2008).
Wright, M. H. et al. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with stem cell characteristics. Breast Cancer Res. 10, R10 (2008).
Pece, S. et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer cell stem content. Cell 140, 62–73 (2010).
Lim, E. et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 15, 907–913 (2009).
Weigelt, B., Peterse, J. L. & van't Veer, L. J. Breast cancer metastasis: markers and models. Nat. Rev. Cancer 5, 591–602 (2005).
Natrajan, R. et al. An integrative genomic and transcriptomic analysis reveals molecular pathways and networks regulated by copy number aberrations in basal-like, HER2 and luminal cancers. Breast Cancer Res. Treat. doi: 10.1007/s10549-009-0501–3.
Holstege, H. et al. High incidence of protein-truncating TP53 mutations in BRCA1-related breast cancer. Cancer Res. 69, 3625–3633 (2009).
Natrajan, R. et al. Tiling path genomic profiling of grade 3 invasive ductal breast cancers. Clin. Cancer Res. 15, 2711–2722 (2009).
Chin, K. et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10, 529–541 (2006).
Adélaïde, J. et al. Integrated profiling of basal and luminal breast cancers. Cancer Res. 67, 11565–11575 (2007).
Stephens, P. J. et al. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462, 1005–1010 (2009).
Cicalese, A. et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell 138, 1083–1095 (2009).
Carey, L. A. et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295, 2492–2502 (2006).
Tilanus-Linthorst, M. et al. A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumor independently contribute to a frequent false-negative mammography. Int. J. Cancer 102, 91–95 (2002).
Evans, A. J. et al. Basal phenotype: a powerful prognostic factor in small screen-detected invasive breast cancer with long-term follow-up. J. Med. Screen. 14, 210–214 (2007).
Robson, M. E. et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 6, R8–R17 (2004).
Kriege, M. et al. Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 27, 3764–3771 (2009).
Acknowledgements
W. D. Foulkes and S. A. Narod are funded by the Canadian Breast Cancer Research Alliance. J. S. Reis-Filho is funded by Breakthrough Breast Cancer, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Foulkes, W., Reis-Filho, J. & Narod, S. Tumor size and survival in breast cancer—a reappraisal. Nat Rev Clin Oncol 7, 348–353 (2010). https://doi.org/10.1038/nrclinonc.2010.39
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2010.39
This article is cited by
-
Value of S-Detect combined with multimodal ultrasound in differentiating malignant from benign breast masses
Egyptian Journal of Radiology and Nuclear Medicine (2024)
-
Machine learning-based models for the prediction of breast cancer recurrence risk
BMC Medical Informatics and Decision Making (2023)
-
Ovarian removal and subsequent breast cancer prognosis: a nationwide cohort study
Breast Cancer Research and Treatment (2023)
-
No significant relationship exists between tumor size and prognosis in distant metastatic hepatocellular carcinoma: a propensity score matching analysis based on SEER database
BMC Gastroenterology (2022)
-
Locoregional tumor burden and risk of mortality in metastatic breast cancer
npj Precision Oncology (2022)