Key Points
-
T-cell epitopes from tumour antigens have been included in many vaccination studies, and their potential to induce antitumour immune responses has become manifest. Nevertheless, the clinical outcome of such studies has to be improved.
-
The number of known epitopes is still limited; therefore, some tumours cannot be treated by immunotherapeutic approaches. For others, the efficacy is not yet optimal.
-
T-cell epitopes from tumour antigens can be defined by two principal strategies: one starts from an existing T-cell response and identifies the target of the response, whereas the other uses the sequence of a tumour antigen and employs epitope prediction to identify the relevant epitopes.
-
New tumour antigens can be discovered by analysing the specificity of existing T-cell responses, or by screening strategies such as the SEREX programme, comparative proteome analysis or gene-expression profiling.
-
To improve the clinical outcome of antitumour vaccinations, tumour-escape mechanisms have to be avoided by the use of efficient, multitarget vaccines.
-
With the growing number of T-cell epitopes, it will become feasible to design patient-specific, individual vaccines that address several different antigens from one tumour and several HLA specificities, including class-II-restricted epitopes.
Abstract
Ten years ago, the first melanoma patient was successfully treated by vaccination with a short peptide, which was, in fact, the first tumour-specific T-cell epitope ever defined — MAGE. Since then, a number of clinical vaccination studies have underlined the potential of tumour-specific T-cell epitopes. But, how can we identify more epitopes to improve their efficacy as an anticancer treatment?
Similar content being viewed by others
Main
The first indication that tumours were immunogenic came from animal models, in which tumours were rejected. There are, however, marked differences in the degree of immunogenicity between different tumours. Some are easily rejected, whereas others escape destruction by the immune system. So, can we use this knowledge to increase tumour immunogenicity as a therapeutic strategy? A large number of pre-clinical and clinical trials in humans have demonstrated the feasibility of antitumour vaccinations. Vaccination is usually well tolerated, which highlights the safety of this approach.
Tumour vaccines make use of tumour antigens — which are, in general, derived from proteins that are produced by the tumour — and aim to trigger a cellular immune response that is executed by T cells. Tumour antigens can be either tumour specific, so are expressed exclusively in tumour tissue, or tumour associated, so are highly overexpressed in tumours but can also be found in normal tissue; differentiation antigens are overexpressed in tumours and are found only in certain types of normal tissue (see below, and Table 1). Peptides from tumour antigens, like those from every other cellular protein, are released from their source proteins during antigen processing, mainly by the proteasome, and are presented by human leukocyte antigen (HLA) class I molecules on the cell surface. Such HLA–peptide complexes are under the surveillance of the cellular immune system. The cellular immune response consists of a cytotoxic effector function, which is carried out by cytotoxic T cells that are positive for the CD8 surface marker and recognize peptides that are presented by HLA class I molecules, and a helper function, which is executed by CD4-positive T cells that are restricted by major histocompatibility complex (MHC) CLASS II MOLECULES (see Box 1). The type of immune reaction that is initiated therefore depends on which MHC molecule presents a peptide from the antigen. During the 1990s, when tumour antigens and their peptide fragments — epitopes, which are presented by MHC molecules — were extensively characterized, much of the work focused on CYTOTOXIC T LYMPHOCYTES (CTLs) and the MHC CLASS I antigen-processing system. In fact, owing to experimental limitations, our knowledge of MHC class-II-restricted T-helper epitopes is still rather limited.
Many epitopes have been reported, some of which are derived from the classical tumour antigens that were identified a long time ago — such as p53, RAS and carcinoembryonic antigen — but others are from recently discovered tumour antigens. More than 70 tumour antigens that are recognized by (mainly CD8+) T cells were listed in a recent review1. In principle, a short peptide that is derived from the sequence of a tumour antigen and presented by an HLA class I molecule represents a potential tumour vaccine. We do not know exactly how many times an individual overcomes tumours during their lifetime. The risk of immunosuppressed individuals developing certain virus-associated tumours — such as non-Hodgkin's lymphoma and Kaposi's sarcoma — is rather high, but, for most kinds of tumour, impaired functions of the immune system do not seem to be important for suppressing tumour formation. Nevertheless, we have to realize that the immune system has failed when tumours develop. This might be because the tumour orginates from 'self' and is therefore not immunogenic. It could also be that the very low number of antigens that are present in the early stages of tumour development does not induce an immune response, as is the case with low doses of pathogens.
The wide variety of antitumour vaccines that have already been applied show that the perfect vaccine — with respect to the selection and number of antigens, and the application strategy — has still to be designed. One of the most favoured applications uses the most efficient professional antigen-presenting cells, DENDRITIC CELLS (DCs), pulsed with peptides from tumour antigens. The number of cells and the number of different — in part, 'engineered' — peptides administered, and the application of adjuvants or enhancers differs among all studies. Although a complete tumour regression has been reported several times2,3, reports of a poor clinical outcome unfortunately also exist4,5, and the first critical opinions have already been voiced; these speak of disappointing trials6. There are several possible reasons for the failure of antitumour vaccination, one of which is loss of the antigen from the tumour, which leads to tumour-escape variants7. But the loss or downregulation of specific molecules in the antigen-processing pathway has also been reported8,9. In addition, immune responses occasionally even fail if antigen, MHC and specific T cells are present, which indicates that tumours can also use other mechanisms to exert a certain form of immunosuppression.
The use of T-cell epitopes in immunotherapy of tumours still presents us with the problem that individual differences between patients — both in the type of HLA molecules and in the epitopes that are presented and recognized — have not yet been finely ascertained. Patient-specific expression of tumour-associated antigens, quantitative differences in HLA-restricted presentation of epitopes and T-cell pools that vary among individuals might all have a significant role. For example, in the SYFPEITHI database10 (see online links box), which lists cancer-related T-cell epitopes, 11 different HLA-A*0201-restricted CTL epitopes from ERBB2 (HER2/neu) are listed, and we do not know which is most efficient in the struggle against an individual tumour (see Box 2). A significant limitation of many clinical trials is their use of one or a few epitopes that is presented by only one HLA restriction element, as this facilitates tumour escape because loss of only one antigen suffices. To promote the exciting option of defeating tumours by 'self', the basic strategies must now be corrected. The future of a cancer immunotherapy that exploits T-cell epitopes from tumour antigens lies in individual, patient-specific analysis and therapy, and in using several tumour antigens and epitopes that are presented by more than two HLA molecules. However, the number of tumour-specific T-cell epitopes that are known today is still too low, with the possible exception of those for malignant MELANOMA, which remains the model system for tumour vaccines. There is an urgent need for more epitopes so that several tumour antigens can be addressed with one vaccine, and to expand the applicability to a larger number of different tumours. So, which strategies will allow us to define new tumour antigens and T-cell epitopes?
Tumour antigens
Tumour immunologists have defined five different classes of antigens that are associated with tumours (Table 1) and that can be targets of antitumour T-cell responses if short peptide fragments that are derived from their sequences are presented by HLA class I molecules. However, we still do not know if most of them are also capable of delivering antigenic peptides to MHC class II molecules. So, what are the characteristics of tumour antigens that make them useful in antitumour vaccines? Most important are tumour-specific antigens: cancer-testis antigens, protein variants that are created by somatic mutations within tumour cells, and proteins from tumour viruses. These are found only in tumour tissue1, and therefore do not encompass the risk of autoimmune reactions during immunotherapy. Antigens that are highly overexpressed in tumours are referred to as tumour associated and might also serve as targets of a cellular immune response. The occurrence of the antigen within the population is also a measure of how successful a vaccine might be. Ubiquitous or shared tumour antigens are expressed by most tumour-bearing individuals and include telomerase11 and survivin12, but antigens that are expressed by only a small percentage of tumours — such as the MAGE gene family13,14,15 — might also be used. So, in present clinical studies, tumour specificity and widespread expression have to be considered to further the development of tumour vaccines. An ideal treatment would include only tumour antigens that are expressed by the respective tumour.
Identifying T-cell epitopes from tumour antigens
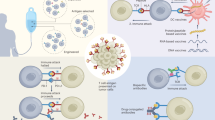
Two experimental strategies are currently being used. One begins with a documented T-cell reaction against tumour cells, and characterizes T-cell specificity and the nature of the antigen that is recognized. The other uses known tumour antigens as its starting point, and uses the 'reverse immunology' approach to predict in silico T-cell epitopes from the sequence of such antigens, according to allele-specific peptide motifs of HLA molecules. T cells are subsequently induced by stimulation with synthetic peptides, and peptide-specific T cells are then tested for the recognition and killing of tumour cells (Fig. 1).
Depending on the starting point, which is either a T-cell reaction (a) or a known tumour antigen (b), two different strategies can be followed. a | The specificity of a T-cell receptor can be characterized using expression libraries, in order to identify the relevant antigen and its T-cell epitope. b | Potential T-cell epitopes can be predicted from the sequence of a tumour antigen. Their ability to induce peptide-specific T cells is assayed, and the tumour specificity of such T cells is verified by assaying tumour-cell recognition. HLA, human leukocyte antigen; MHC, major histocompatibility complex.
From T-cell recognition to T-cell epitopes. The classical approach is to investigate the specificity of T cells that recognize tumour cells in order to define the epitope (Fig. 1a). The first tumour-specific T-cell epitope that was identified was from MAGE1 (Ref. 16), and was presented by HLA-A*0101. It was defined by narrowing down the complexity of antigens that produced the immune response in the target melanoma cell. Thierry Boon and colleagues expressed human cDNA libraries that were generated from melanoma cells, in COS cells that were transfected with the HLA-A*0101 gene, until the T-cell reaction focused on a single gene. Using truncation experiments, a small region of DNA was identified that harboured the relevant T-cell recognition site17. Since then, the methods for expression cloning have been substantially improved; a very efficient expression library has recently been described, which enables identification of T-cell specificity after enrichment steps18.
An alternative approach, which also starts from T-cell function, uses biochemical methods to purify MHC molecules that present the relevant peptide epitope. After acid elution from the MHC molecule, the highly complex mixture of MHC ligands is fractionated by chromatography (capillary high-performance liquid chromatography) and the fraction that is recognized by T cells is then analysed by mass spectrometry19. The low number of tumour-specific peptides among thousands of inconspicuous peptides, the often low affinity of T cells for tumour epitopes, and the very low number of peptides, impede this molecular characterization. Less than 10 picograms of peptide have to be analysed, which requires highly sensitive analytical techniques. This strategy is very tedious and risky, for all these reasons, and such cases remain rare exceptions in tumour-epitope characterization.
From antigens to T-cell epitopes. Immediately after publication of the first HLA-allele-specific peptide motifs20, scientists used the motifs to predict T-cell epitopes from antigen sequences21,22. This approach has been termed 'reverse immunology'23 and usually starts from a known tumour antigen, but without pre-existing T cells (Fig. 1b). Epitope prediction has now been carried out for more than ten years, and most tumour-specific T-cell epitopes known today have been defined with the help of peptide motifs. Several programmes offer free epitope predictions for a wide range of MHC class-I- and class-II-restricted epitopes from different organisms — the most commonly used are BIMAS24 and SYFPEITHI. In addition, programmes have evolved during the past two years that offer the prediction of proteasomal cleavages25,26: an overview on prediction programmes is given in Ref. 27. The experimental verification of proteasomal cleavage sites28 or the selected potential epitopes starts with peptide synthesis. Synthetic peptides might be used to immunize transgenic mice to produce a T-cell response29, or to re-stimulate precursor T cells in vitro, which are derived from either healthy blood donors or tumour patients30. The final step to establishing tumour-specific T cells that have a distinct specificity is accomplished when the raised T cells recognize tumour cells that express the respective antigen. A direct demonstration of natural processing of the predicted epitopes can be achieved by the 'predict, calibrate and detect' method31, which uses synthetic peptide candidates as calibrants for a capillary-chromatography–mass-spectrometric system, and by comparing the results with those obtained after acid elution of naturally processed peptides from HLA molecules of tumour cells.
New antigens as a source of new epitopes
With the development of screening methods to identify tumour-associated antigens, huge data sets had to be evaluated. The first screening method, SEREX, uses antibodies from sera of tumour patients to determine potential tumour antigens32: all proteins recognized by such antibodies (but not by antibodies from healthy persons) have been included in the SEREX database (see online links box), which now contains many hundreds of antigens. Two other screening methods — PROTEOMICS33,34,35 and MICROARRAY ANALYSIS36,37 — investigate the differences between healthy tissue and tumour tissue at the protein or mRNA level, respectively. With this type of differential analysis, several dozen to several hundred tumour-associated antigens can be defined within one assay, depending on the experimental thresholds. After screening a series of samples, scientists need to interpret large amounts of data in the light of published work and database contents. Results from complementary methods have to be combined to obtain reliable information. For example, the combination of differential display at the protein level, and the recognition of antigens by patients' antibodies might reveal interesting candidate antigens38, as well as the combination of expression profiling using DNA microarrays with the analysis of peptides presented by HLA molecules of the same tumour sample. After establishing a new tumour-associated antigen, the search for T-cell epitopes from this antigen might start anew by employing reverse immunology36.
Help is required
Vaccinations that are aimed at recruiting cytotoxic T cells for tumour destruction or prevention have been successful but, unfortunately, the success has been limited to just a few cases. The response rates that have been documented in many clinical studies are encouraging, but must be increased. In addition to tumour-escape mechanisms, inefficient triggering of the T-cell system might be a reason for low response rates. In order to mimic the concerted action of the immune system against pathogens, epitopes that stimulate CD4+ T-helper cells have recently been included in antitumour peptide vaccination studies39,40,41. Ideally, the epitopes that are recognized by CTLs and T-helper cells should be derived from the same tumour antigen. However, although many tumour-specific CTL epitopes are known, only a very small number of HLA class-II-presented, tumour-specific T-helper epitopes have been characterized. Increased efforts are therefore being made to identify tumour-associated epitopes that are presented by class II molecules42,43,44,45, which would activate CD4+ helper cells to induce and maintain CD8+ killer cells46. In principle, the strategies that are used for the characterization of CTL epitopes can also be applied to helper epitopes. This holds true for the classical approach in particular, which starts from T-cell recognition, although transfection of MHC class-II-expressing antigen-presenting cells with expression libraries represents a specific problem. An elegant strategy directs the antigen of interest to the lysosomal/endosomal compartment, where antigen processing leads to the generation of MHC class-II-presented epitopes47,48,49,50. Performing reverse immunology for MHC class-II-restricted antigen presentation is far more difficult, for two reasons. First, the peptide motifs of MHC class II molecules are more degenerate compared with class I motifs and therefore do not allow for similarly precise predictions. Second, it is impossible, at present, to predict the proteolytic events that take place during antigen processing within the MHC class II pathway.
Diverse clinical applications: partial success
The first vaccination that was carried out with a tumour-specific T-cell epitope was in a melanoma patient. Since then, melanoma has represented the model system for cancer immunotherapy. So far, most T-cell epitopes from tumour antigens have been derived from melanomal or MELANOSOMAL proteins. With the growing number of T-cell epitopes identified, the studies have now been expanded to other tumours. Clinical studies have been carried out in more then ten tumour entities, including cancers of the breast, cervix, gastrointestinal duct, lung, ovary, pancreas, prostate, kidney and medullary thyroid.
Although many cancer patients have been treated successfully, these represent just a small percentage of all individuals who underwent vaccine therapy (Table 2). Most patients that were included in clinical studies did not benefit from treatment with peptide epitopes that were identified from tumour antigens. We can speculate about the possible reasons that lead to such a disappointing outcome, but it is important to keep in mind that terminal patients — who have already undergone therapies in which their tumours proved to be resistant — are most frequently included in trials. It would be very interesting to compare these results with a first-line immunotherapeutic treatment of low-risk patients.
An additional factor is the individual differences between people (Box 2), which have never been assessed in their entirety. We are also still unsure of the optimal application of tumour-associated T-cell epitopes: the many possible methods of vaccination have never been compared systematically in humans. Even if we leave out viral or other vectors, proteins and DNA, and only consider the application of synthetic peptides, each protocol that has been applied — use of PERIPHERAL-BLOOD MONONUCLEAR CELLS (PBMCs) or DCs as carriers; peptide only, intradermal or subcutanous administration; or addition of different adjuvants, cytokines or helper epitopes — has a limited, but comparable success (Table 2). It is therefore impossible to state that one thing works and another does not. Most application protocols have been shown to induce T-cell responses in patients, but tumour regression has been observed in only a few cases.
New approaches, future directions
There is much scope for the successful application of tumour-associated T-cell epitopes in cancer therapy, as the proof of principle was established long ago: immune responses are induced in most cases, and most tumour types are potential targets of immunotherapy. The problems, however, include the unsolved question of optimal clinical application, unknown escape mechanisms of tumours, and the low number of T-cell epitopes that have been identified for non-melanoma tumours. It has often been argued that HLA polymorphisms are a significant drawback in tumour immunology, but this is not as hopeless as it might seem at first glance: the most abundant HLA molecules have been investigated thoroughly, and their peptide motifs or even crystal structures have been determined. Our knowledge includes peptide motifs of HLA molecules that are expressed in more than 95% of Caucasians.
Activities must now focus on the identification of more tumour-associated antigens, and T-cell epitopes that are derived from their sequences. Fig. 2 shows how many different methods can be used to obtain complementary information about the characteristics of a given tumour. The combination of proteomics and antibody screening has been reported38, and the combination of expression profiling, HLA-ligand characterization and T-cell assays from blood has been established in our own department (Weinschenk, T. et al., manuscript in preparation). A modern anticancer vaccine should not target one or a few antigens only. It is also important to leave the model system of HLA-A*0201, and to characterize T-cell epitopes that can be presented by many different HLA class I or class II molecules. The most promising strategy seems to be an individual analysis of every tumour that is accessible, and individual treatment of every tumour patient (see also Box 2 and Fig. 2). The modern peptide vaccine should consist of tumour-associated CTL epitopes and helper epitopes from various tumour antigens that are restricted to more than two HLA class I molecules and one HLA class II molecule. Hopefully, these developments will lead to real benefits for patients with cancer.
As much information as possible has to be compiled about the presence of antigens or epitopes in a given tumour in order to identify new tumour antigens, and new tumour-associated T-cell epitopes that can be used in clinical studies. This strategy could lead to the characterization of an individual, patient-specific T-cell epitope pattern, which represents the prescription to treat this tumour. HLA, human leukocyte antigen.
References
Renkvist, N., Castelli, C., Robbins, P. F. & Parmiani, G. A listing of human tumor antigens recognized by T cells. Cancer Immunol. Immunother. 50, 3–15 (2001).
Nestle, F. O. et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nature Med. 4, 328–332 (1998).
Rosenberg, S. A. et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature Med. 4, 321–327 (1998).
Lee, K. H. et al. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. J. Immunol. 163, 6292–6300 (1999).
Panelli, M. C. et al. Phase I study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART1 and GP100. J. Immunother. 23, 487–498 (2000).
Bodey, B., Bodey, B. Jr, Siegel, S. E. & Kaiser, H. E. Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res. 20, 2665–2676 (2000).
Ohnmacht, G. A. et al. Short-term kinetics of tumor antigen expression in response to vaccination. J. Immunol. 167, 1809–1820 (2001).
Tait, B. D. HLA class I expression on human cancer cells. Implications for effective immunotherapy. Hum. Immunol. 61, 158–165 (2000).
Seliger, B. et al. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 61, 8647–8650 (2001).
Rammensee, H.-G., Bachmann, J., Emmerich, N., Bachor, O. A. & Stevanovic, S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50, 213–219 (1999).
Vonderheide, R. H. et al. Characterization of HLA-A3-restricted cytotoxic T lymphocytes reactive against the widely expressed tumor antigen telomerase. Clin. Cancer Res. 7, 3343–3348 (2001).An actual example of reverse immunology, using a ubiquitous tumour antigen, telomerase. Epitope identification was achieved after induction of peptide-specific T cells.
Schmitz, M. et al. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 60, 4845–4849 (2000).
Mashino, K. et al. Expression of multiple cancer-testis antigen genes in gastrointestinal and breast carcinomas. Br. J. Cancer 85, 713–720 (2001).
Gillespie, A. M. et al. MAGE, BAGE and GAGE: tumour antigen expression in benign and malignant ovarian tissue. Br. J. Cancer 78, 816–821 (1998).
Russo, V. et al. Expression of the MAGE gene family in primary and metastatic human breast cancer: implications for tumor antigen-specific immunotherapy. Int. J. Cancer 64, 216–221 (1995).
van der Bruggen, P. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 254, 1643–1647 (1991).
Traversari, C. et al. A nonapeptide encoded by human gene MAGE1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J. Exp. Med. 176, 1453–1457 (1992).
Smith, E. S. et al. Lethality-based selection of recombinant genes in mammalian cells: application to identifying tumor antigens. Nature Med. 7, 967–972 (2001).A new generation of expression libraries that comprises enrichment steps.
Cox, A. L. et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science 264, 716–719 (1994).
Falk, K., Rötzschke, O., Stevanovic, S., Jung, G. & Rammensee, H.-G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351, 290–296 (1991).
Rötzschke, O. et al. Exact prediction of a natural T cell epitope. Eur. J. Immunol. 21, 2891–2894 (1991).
Stevanovic, S. & Rammensee, H.-G. Identification of T-cell epitopes using allele-specific ligand motifs. Behring. Inst. Mitt. 95, 7–13 (1994).
Celis, E. et al. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc. Natl Acad. Sci. USA 91, 2105–2109 (1994).
Parker, K. C., Bednarek, M. A. & Coligan, J. E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152, 163–175 (1994).
Nussbaum, A. K. et al. PapRoC: a predicition algorithm for proteasomal cleavages available on the WWW. Immunogenetics 53, 87–94 (2001).
Kesmir, C. et al. 〈http://www.cbs.dtu.dk/services/NetChop/〉.
Schirle, M., Weinschenk, T. & Stevanovic, S. Combining computer algorithms with experimental approaches permits the rapid and accurate identification of T cell epitopes from defined antigens. J. Immunol. Methods 257, 1–16 (2001).
Kessler, J. H. et al. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J. Exp. Med. 193, 73–88 (2001).Use of proteasomal digestion for the definition of HLA-presented peptides from tumour antigens.
Pascolo, S. et al. A MAGE-A1 HLA-A*0201 epitope identified by mass spectrometry. Cancer Res. 61, 4072–4077 (2001).Identification of a tumour-specific class I epitope by mass spectrometry without the use of pre-existing T cells.
Brossart, P. et al. Identification of HLA-A2 restricted T cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood 93, 4309–4317 (1999).
Schirle, M. et al. Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. Eur. J. Immunol. 30, 2216–2225 (2000).
Sahin, U. et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl Acad. Sci. USA 92, 11810–11813 (1995).
Czerwenka, K. F. et al. Comparative analysis of two-dimensional protein patterns in malignant and normal human breast tissue. Cancer Detect. Prev. 25, 268–279 (2001).
Meehan, K. L., Holland, J. W. & Dawkins, H. J. Proteomic analysis of normal and malignant prostate tissue to identify novel proteins lost in cancer. Prostate 50, 54–63 (2002).
Emmert-Buck, M. R. et al. An approach to proteomic analysis of human tumors. Mol. Carcinog. 27, 158–165 (2000).
Mathiassen, S., Lauemöller, S. L., Ruhwald, M., Claesson, M. H. & Buus, S. Tumor-associated antigens identified by mRNA expression profiling induce protective anti-tumor immunity. Eur. J. Immunol. 31, 1239–1246 (2001).Candidate antigens from gene-expression profiling were used for epitope prediction, leading to protective T-cell epitopes in a mouse model.
Boer, J. M. et al. Identification and classification of differentially expressed genes in renal cell carcinoma by expression profiling on a global human 31,500-element cDNA array. Genome Res. 11, 1861–1870 (2001).
Klade C. S. et al. Identification of tumor antigens in renal cell carcinoma by serological proteome analysis. Proteomics 1, 890–898 (2001).
van Driel, W. J. et al. Vaccination with HPV16 peptides of patients with advanced cervical carcinoma: clinical evaluation of a Phase I–II trial. Eur. J. Cancer 35, 946–952 (1999).
Slingluff, C. L. Jr et al. Phase I trial of a melanoma vaccine with GP100(280–288) peptide and tetanus helper peptide in adjuvant: immunologic and clinical outcomes. Clin. Cancer Res. 7, 3012–3024 (2001).
Brossart, P. et al. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood 96, 3102–3108 (2000).
Kobayashi, H., Song, Y., Hoon, D. S., Appella, E. & Celis, E. Tumor-reactive T helper lymphocytes recognize a promiscuous MAGE-A3 epitope presented by various major histocompatibility complex class II alleles. Cancer Res. 61, 4773–4778 (2001).A recent example of how to identify tumour-specific HLA class-II-presented epitopes.
Schultz, E. S. et al. A MAGE-A3 peptide presented by HLA-DP4 is recognized on tumor cells by CD4+ cytolytic T lymphocytes. Cancer Res. 60, 6272–6275 (2000).
Kobayashi, H., Lu, J. & Celis, E. Identification of helper T-cell epitopes that encompass or lie proximal to cytotoxic T-cell epitopes in the GP100 melanoma tumor antigen. Cancer Res. 61, 7577–7584 (2001).
Chaux, P. et al. MAGE1 peptide recognized on HLA-DR15 by CD4+ T cells. Eur. J. Immunol. 31, 1910–1916 (2001).
Casares, N. et al. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated TH1 helper peptide elicits protective CTL immunity. Eur. J. Immunol. 31, 1780–1789 (2001).
Marks, M. S. et al. A lysosomal targeting signal in the cytoplasmic tail of the β-chain directs HLA-DM to MHC class II compartments. J. Cell Biol. 131, 351–369 (1995).
Sanderson, S., Frauwirth, K. & Shastri, N. Expression of endogenous peptide–major histocompatibility complex class II complexes derived from invariant chain-antigen fusion proteins. Proc. Natl Acad. Sci. USA 92, 7217–7221 (1995).
Malcherek, G. et al. MHC class II-associated invariant chain peptide replacement by T cell epitopes: engineered invariant chain as a vehicle for directed and enhanced MHC class II antigen processing and presentation. Eur. J. Immunol. 28, 1524–1533 (1998).
Rodriguez, F., Harkins, S., Redwine, J. M., de Pereda, J. M. & Whitton, J. L. CD4+ T cells induced by a DNA vaccine: immunological consequences of epitope-specific lysosomal targeting. J. Virol. 75, 10421–10430 (2001).
van der Bruggen, P. et al. A peptide encoded by human gene MAGE3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE3. Eur. J. Immunol. 24, 3038–3043 (1994).
Jäger, E. et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J. Exp. Med. 187, 265–270 (1998).
Wölfel, T. et al. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269, 1281–1284 (1995).
Robbins, P. F. et al. A mutated β-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J. Exp. Med. 183, 1185–1192 (1996).
Mandruzzato, S., Stroobant, V., Demotte, N. & van der Bruggen, P. A human CTL recognizes a caspase-8-derived peptide on autologous HLA-B*3503 molecules and two unrelated peptides on allogeneic HLA-B*3501 molecules. J. Immunol. 164, 4130–4134 (2000).
Ressing, M. E. et al. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J. Immunol. 154, 5934–5943 (1995).
Brichard, V. G. et al. A tyrosinase nonapeptide presented by HLA-B44 is recognized on a human melanoma by autologous cytolytic T lymphocytes. Eur. J. Immunol. 26, 224–230 (1996).
Skipper, J. C. et al. Shared epitopes for HLA-A3-restricted melanoma-reactive human CTL include a naturally processed epitope from PMEL17/GP100. J. Immunol. 157, 5027–5033 (1996).
Salgaller, M. L. et al. Dendritic cell-based immunotherapy of prostate cancer. Crit. Rev. Immunol. 18, 109–119 (1998).
Fisk, B., Blevins, T. L., Wharton, J. T. & Ioannides, C. G. Identification of an immunodominant peptide of HER2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J. Exp. Med. 181, 2109–2117 (1995).
Kawashima, I. et al. Identification of HLA-A3-restricted cytotoxic T lymphocyte epitopes from carcinoembryonic antigen and HER2/neu by primary in vitro immunization with peptide-pulsed dendritic cells. Cancer Res. 59, 431–435 (1999).
Scheibenbogen, C. et al. Phase 2 trial of vaccination with tyrosinase peptides and granulocyte-macrophage colony-stimulating factor in patients with metastatic melanoma. J. Immunother. 23, 275–281 (2000).
Hunger, R. E. et al. Successful induction of immune responses against mutant RAS in melanoma patients using intradermal injection of peptides and GM–CSF as adjuvant. Exp. Dermatol. 10, 161–167 (2001).
Mackensen, A. et al. Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Int. J. Cancer 86, 385–392 (2000).
Yu, J. S. et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 61, 842–847 (2001).
Gjertsen, M. K. et al. Intradermal RAS peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: clinical and immunological responses in patients with pancreatic adenocarcinoma. Int. J. Cancer 92, 441–450 (2001).
Gajewski, T. F., Fallarino, F., Ashikari, A. & Sherman, M. Immunization of HLA-A2+ melanoma patients with MAGE3 or MelanA peptide-pulsed autologous peripheral blood mononuclear cells plus recombinant human interleukin 12. Clin. Cancer Res. 7, 895s–901s (2001).
Schott, M. et al. Immunotherapy for medullary thyroid carcinoma by dendritic cell vaccination. J. Clin. Endocrinol. Metab. 86, 4965–4969 (2001).
Jäger, E. et al. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc. Natl Acad. Sci. USA 97, 12198–12203 (2000).
Rosenberg, S. A. et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J. Immunol. 163, 1690–1695 (1999).
Murphy, G. P. et al. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate 38, 73–78 (1999).
Sadanaga, N. et al. Dendritic cell vaccination with MAGE peptide is a novel therapeutic approach for gastrointestinal carcinomas. Clin. Cancer Res. 7, 2277–2284 (2001).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Medscape DrugInfo
FURTHER INFORMATION
Proteasomal cleavage prediction NetChop
Glossary
- HLAs
-
Human leukocyte antigens, which are molecules of the human major histocompatibility complex.
- CD8
-
A surface molecule that is expressed exclusively by cytotoxic T cells.
- CD4
-
A surface molecule that is expressed exclusively by T-helper cells.
- MHC CLASS II MOLECULES
-
Peptide receptors, similar to class I molecules in structure and function, but exclusively expressed by a small set of immune cells. They mainly present peptides from extracellular proteins to T-helper cells.
- CYTOTOXIC T LYMPHOCYTES
-
(CTLs: killer cells, cytotoxic T cells). These control all major histocompatibility complex class-I-expressing body cells for the presence of abnormal (viral, tumour-associated) peptides.
- MHC CLASS I MOLECULES
-
Highly polymorphic glycoproteins that are expressed by every nucleated body cell of vertebrates, and that are encoded by the gene cluster 'major histocompatibility complex' (MHC). The human MHC molecules are termed HLA (human leukocyte antigen) molecules. MHC class I molecules mainly present peptides from intracellular proteins to cytotoxic T cells.
- DENDRITIC CELLS
-
(DCs). These present T-cell epitopes very efficiently and are able to activate cytotoxic T lymphocytes.
- MELANOMA
-
Skin cancer, originating from transformed melanocytes.
- MAGE
-
Melanoma antigen; a family of proteins that are expressed only in testis or tumour cells.
- PROTEOMICS
-
Analysis of the entirety of proteins from a tissue sample, separated by two-dimensional gel electrophoresis. Each individual protein spot can be identified after tryptic digestion and mass spectrometrical analysis of the resulting peptides.
- MICROARRAY ANALYSIS
-
'DNA chips' contain many thousands of oligonucleotides that represent fragments of genes, immobilized on a solid surface. They are probed with cDNA that is prepared from mRNA of tissue samples and so give quantitative information about gene expression.
- MELANOSOMES
-
Subcellular organelles in melanocytes, which contain the skin's pigments.
- PERIPHERAL-BLOOD MONONUCLEAR CELLS
-
(PBMCs). Includes B cells, T cells and monocytes. These can be obtained from whole blood after ficoll density-gradient centrifugation.
Rights and permissions
About this article
Cite this article
Stevanovic, S. Identification of tumour-associated t-cell epitopes for vaccine development. Nat Rev Cancer 2, 514 (2002). https://doi.org/10.1038/nrc841
Issue Date:
DOI: https://doi.org/10.1038/nrc841
This article is cited by
-
VaccImm: simulating peptide vaccination in cancer therapy
BMC Bioinformatics (2013)
-
Profound tumor-specific Th2 bias in patients with malignant glioma
BMC Cancer (2012)
-
Poly(I:C)-induced tumour cell death leads to DC maturation and Th1 activation
Cancer Immunology, Immunotherapy (2011)
-
CpG-conjugated apoptotic tumor cells elicit potent tumor-specific immunity
Cancer Immunology, Immunotherapy (2011)
-
Regulatory T Cell as a Target for Cancer Therapy
Archivum Immunologiae et Therapiae Experimentalis (2010)