Key Points
-
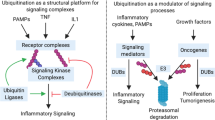
Ubiquitin is an 8 kDa polypeptide that can be covalently attached to other proteins through a process called ubiquitylation (also known as ubiquitination). Similar to phoshporylation, ubiquitylation is an inducible and reversible process that changes the properties of the modified substrate; for example, its subcellular localization, stability or enzymatic activity.
-
The attachment of ubiquitin to substrates requires the sequential action of three enzymes: ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin ligase (E3). This process can be reversed by de-ubiquitylating enzymes (DUBs) that remove ubiquitin from substrates.
-
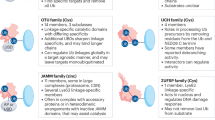
Several ubiquitin-like modifiers (Ubls) have been identified, such as SUMO, NEDD8, ISG15 and FAT10. They possess a distinct primary sequence but share characteristic features with ubiquitin, including the 3-dimensional fold and the mode of their conjugation to substrates.
-
Protein domains that bind to ubiquitin (UBDs) or Ubls have crucial roles in the recognition of modified proteins and the translation of the encoded information into the proper cellular response. Most UBD-containing proteins undergo monoubiquitylation themselves, which causes their auto-inhibition by intramolecular UBD–ubiquitin interactions.
-
Alterations to the ubiquitin and Ubl systems are associated with various pathologies, such as inflammatory diseases and cancer. Moreover, many disease-related pathways are not only regulated by a single ubiquitin or Ubl, but by a sophisticated cross-talk of different types of modifications.
-
Examples of such ubiquitin or Ubl interplays in oncogenic pathways include p53, nuclear factor κB and DNA repair pathways, which are regulated by monoubiquitylation, polyubiquitylation, sumoylation and neddylation.
-
Bortezomib is the first compound that targets the ubiquitin-system to be approved for clinical use in human cancers. It inhibits the active site of one of the proteasome subunits and shuts down the proteasomal protein-degradation system.
-
New, more specific anticancer drugs that target the ubiquitin-system are being developed, including selective inhibitors of E3 enzymes like MDM2. Moreover, DUBs and the binding interface of ubiquitylated proteins and UBDs have emerged as promising drug targets.
Abstract
Ubiquitin and ubiquitin-like proteins (Ubls) are signalling messengers that control many cellular functions, such as cell proliferation, apoptosis, the cell cycle and DNA repair. It is becoming apparent that the deregulation of ubiquitin pathways results in the development of human diseases, including many types of tumours. Here we summarize the common principles and specific features of ubiquitin and Ubls in the regulation of cancer-relevant pathways, and discuss new strategies to target ubiquitin signalling in drug discovery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Pickart, C. M. & Fushman, D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8, 610–616 (2004).
Haglund, K. & Dikic, I. Ubiquitylation and cell signaling. EMBO J. 24, 3353–3359 (2005).
Di Fiore, P. P., Polo, S. & Hofmann, K. When ubiquitin meets ubiquitin receptors: a signalling connection. Nature Rev. Mol. Cell Biol. 4, 491–497 (2003).
Hecker, C. M., Rabiller, M., Haglund, K., Bayer, P. & Dikic, I. Specification of SUMO1- and SUMO2-interacting Motifs. J. Biol. Chem. 281, 16117–16127 (2006).
Hicke, L., Schubert, H. L. & Hill, C. P. Ubiquitin-binding domains. Nature Rev. Mol. Cell Biol. 6, 610–621 (2005).
Song, J., Durrin, L. K., Wilkinson, T. A., Krontiris, T. G. & Chen, Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl Acad. Sci. USA 101, 14373–14378 (2004).
Nakayama, K. I. & Nakayama, K. Ubiquitin ligases: cell-cycle control and cancer. Nature Rev. Cancer 6, 369–381 (2006).
Brooks, C. L. & Gu, W. p53 ubiquitination: Mdm2 and beyond. Mol. Cell 21, 307–315 (2006).
Cardozo, T. & Pagano, M. The SCF ubiquitin ligase: insights into a molecular machine. Nature Rev. Mol. Cell Biol. 5, 739–751 (2004).
Schmidt, M. H. & Dikic, I. The Cbl interactome and its functions. Nature Rev. Mol. Cell Biol. 6, 907–919 (2005).
Thien, C. B. & Langdon, W. Y. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem. J. 391, 153–166 (2005).
Nijman, S. M. et al. A genomic and functional inventory of deubiquitinating enzymes. Cell 123, 773–786 (2005).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Alarcon-Vargas, D. & Ronai, Z. SUMO in cancer-wrestlers wanted. Cancer Biol. Ther. 1, 237–242 (2002).
D'Cunha, J., Knight, E. Jr., Haas, A. L., Truitt, R. L. & Borden, E. C. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc. Natl Acad. Sci. USA 93, 211–215 (1996).
Bode, A. M. & Dong, Z. Post-translational modification of p53 in tumorigenesis. Nature Rev. Cancer 4, 793–805 (2004).
Vousden, K. H. & Prives, C. P53 and prognosis: new insights and further complexity. Cell 120, 7–10 (2005).
Xirodimas, D. P., Stephen, C. W. & Lane, D. P. Cocompartmentalization of p53 and Mdm2 is a major determinant for Mdm2-mediated degradation of p53. Exp. Cell Res. 270, 66–77 (2001).
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003).
Erster, S., Mihara, M., Kim, R. H., Petrenko, O. & Moll, U. M. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol. Cell. Biol. 24, 6728–6741 (2004).
Mihara, M. & Moll, U. M. Detection of mitochondrial localization of p53. Methods Mol. Biol. 234, 203–209 (2003).
Mihara, M. et al. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11, 577–590 (2003).
Dumont, P., Leu, J. I., Della Pietra, A. C. III, George, D. L. & Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nature Genet. 33, 357–365 (2003).
Kulikov, R., Winter, M. & Blattner, C. Binding of P53 to the central domain of MDM2 is regulated by phosphorylation. J. Biol. Chem. 26 July 2006 [epub ahead of print].
Ma, J. et al. A second p53 binding site in the central domain of mdm2 is essential for p53 ubiquitination. Biochemistry 45, 9238–9245 (2006).
Dawson, S., Higashitsuji, H., Wilkinson, A. J., Fujita, J. & Mayer, R. J. Gankyrin: a new oncoprotein and regulator of pRb and p53. Trends Cell Biol. 16, 229–233 (2006).
Higashitsuji, H. et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell 8, 75–87 (2005).
Weger, S., Hammer, E. & Heilbronn, R. Topors acts as a SUMO-1 E3 ligase for p53 in vitro and in vivo. FEBS Lett. 579, 5007–5012 (2005).
Rodriguez, M. S. et al. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 18, 6455–6461 (1999).
Gostissa, M. et al. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 18, 6462–6471 (1999).
Bischof, O. et al. The E3 SUMO ligase PIASy is a regulator of cellular senescence and apoptosis. Mol. Cell 22, 783–794 (2006).
Nelson, V., Davis, G. E. & Maxwell, S. A. A putative protein inhibitor of activated STAT (PIASy) interacts with p53 and inhibits p53-mediated transactivation but not apoptosis. Apoptosis 6, 221–234 (2001).
Schmidt, D. & Muller, S. Members of the PIAS family act as SUMO ligases for c-Jun and p53 and repress p53 activity. Proc. Natl Acad. Sci. USA 99, 2872–2877 (2002).
Chen, L. & Chen, J. MDM2–ARF complex regulates p53 sumoylation. Oncogene 22, 5348–5357 (2003).
Xirodimas, D. P., Saville, M. K., Bourdon, J. C., Hay, R. T. & Lane, D. P. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell 118, 83–97 (2004).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002).
Lim, S. K., Shin, J. M., Kim, Y. S. & Baek, K. H. Identification and characterization of murine mHAUSP encoding a deubiquitinating enzyme that regulates the status of p53 ubiquitination. Int. J. Oncol. 24, 357–364 (2004).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53-Mdm2 pathway. Mol. Cell 13, 879–886 (2004). The authors show that the de-ubiquitylating enzyme HAUSP functions on both p53 and the E3 ligase MDM2 that targets p53 for degradation. The study suggests that the cellular level of HAUSP defines the balance between E3 and its substrate p53, and therefore the transcriptional activity of p53.
Cummins, J. M. & Vogelstein, B. HAUSP is required for p53 destabilization. Cell Cycle 3, 689–692 (2004).
Lind, H., Zienolddiny, S., Ekstrom, P. O., Skaug, V. & Haugen, A. Association of a functional polymorphism in the promoter of the MDM2 gene with risk of nonsmall cell lung cancer. Int. J. Cancer 119, 718–721 (2006).
Masuya, D. et al. The HAUSP gene plays an important role in non-small cell lung carcinogenesis through p53-dependent pathways. J. Pathol. 208, 724–732 (2006).
Menin, C. et al. Association between MDM2–SNP309 and age at colorectal cancer diagnosis according to p53 mutation status. J. Natl Cancer Inst. 98, 285–288 (2006).
Karin, M. & Greten, F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 5, 749–759 (2005).
Nakanishi, C. & Toi, M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nature Rev. Cancer 5, 297–309 (2005).
Chen, Z. J. Ubiquitin signalling in the NF-κB pathway. Nature Cell Biol. 7, 758–765 (2005).
Sun, L., Deng, L., Ea, C. K., Xia, Z. P. & Chen, Z. J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Zhou, H. et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 (2004).
Huang, T. T., Wuerzberger-Davis, S. M., Wu, Z. H. & Miyamoto, S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-κB activation by genotoxic stress. Cell 115, 565–576 (2003).
Janssens, S., Tinel, A., Lippens, S. & Tschopp, J. PIDD mediates NF-κB activation in response to DNA damage. Cell 123, 1079–1092 (2005).
Wuerzberger-Davis, S. M., Nakamura, Y., Seufzer, B. J. & Miyamoto, S. NF-κB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene 24 July 2006 [epub ahead of print].
Mabb, A. M., Wuerzberger-Davis, S. M. & Miyamoto, S. PIASy mediates NEMO sumoylation and NF-κB activation in response to genotoxic stress. Nature Cell Biol. 8, 986–993 (2006).
Wu, Z. H., Shi, Y., Tibbetts, R. S. & Miyamoto, S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science 311, 1141–1146 (2006).
Desterro, J. M., Rodriguez, M. S. & Hay, R. T. SUMO-1 modification of IκBα inhibits NF-κB activation. Mol. Cell 2, 233–239 (1998).
Evans, P. C. et al. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem. J. 378, 727–734 (2004).
Heyninck, K. & Beyaert, R. A20 inhibits NF-κB activation by dual ubiquitin-editing functions. Trends Biochem. Sci. 30, 1–4 (2005).
He, K. L. & Ting, A. T. A20 inhibits tumor necrosis factor (TNF) α-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Mol. Cell. Biol. 22, 6034–6045 (2002).
Jaattela, M., Mouritzen, H., Elling, F. & Bastholm, L. A20 zinc finger protein inhibits TNF and IL-1 signaling. J. Immunol. 156, 1166–1173 (1996).
Bignell, G. R. et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nature Genet. 25, 160–165 (2000).
Massoumi, R., Chmielarska, K., Hennecke, K., Pfeifer, A. & Fassler, R. Cyld inhibits tumor cell proliferation by blocking bcl-3-dependent NF-κB signaling. Cell 125, 665–677 (2006). The analysis of CYLD knockout mice reveals BCL3 as a new target of CYLD de-ubiquitylating activity. Therefore, this study suggests that CYLD negatively regulates different NFκB pathways depending on the external signal.
Hashimoto, K. et al. Analysis of DNA copy number aberrations in hepatitis C virus-associated hepatocellular carcinomas by conventional CGH and array CGH. Mod. Pathol. 17, 617–622 (2004).
Hirai, Y. et al. Conventional and array-based comparative genomic hybridization analyses of novel cell lines harboring HPV18 from glassy cell carcinoma of the uterine cervix. Int. J. Oncol. 24, 977–986 (2004).
Strobel, P. et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am. J. Surg. Pathol. 26, 119–124 (2002).
Brummelkamp, T. R., Nijman, S. M., Dirac, A. M. & Bernards, R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003).
Kovalenko, A. et al. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature 424, 801–805 (2003).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424, 793–796 (2003).
Reiley, W., Zhang, M., Wu, X., Granger, E. & Sun, S. C. Regulation of the deubiquitinating enzyme CYLD by IκB kinase gamma-dependent phosphorylation. Mol. Cell. Biol. 25, 3886–3895 (2005).
Friedberg, E. C., Lehmann, A. R. & Fuchs, R. P. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol.Cell 18, 499–505 (2005).
Kannouche, P. L., Wing, J. & Lehmann, A. R. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14, 491–500 (2004). The authors show that UV irradiation induces PCNA monoubiquitylation, which is associated with the recruitment of Y-family polymerases to replication foci and required for the initiation of translesion synthesis.
Kannouche, P. L. & Lehmann, A. R. Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle 3, 1011–1013 (2004).
Haracska, L., Unk, I., Prakash, L. & Prakash, S. Ubiquitylation of yeast proliferating cell nuclear antigen and its implications for translesion DNA synthesis. Proc. Natl Acad. Sci. USA 103, 6477–6482 (2006).
Bienko, M. et al. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 (2005). In this paper two new ubiquitin-binding domains (UBM and UBZ) are identified that are conserved in TLS polymerases and mediate the binding to monoubiquitylated PCNA after UV-induced DNA damage.
Plosky, B. S. et al. Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J. 25, 2847–2855 (2006).
Huang, T. T. et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nature Cell Biol. 8, 341–347 (2006). The authors discover a mechanism that enables the accumulation of monoubiquitylated PCNA after UV-induced DNA damage. This mechanism involves the signal-induced auto-inactivation of the de-ubiquitylating enzyme USP1.
Miller, S. L., Malotky, E. & O'Bryan, J. P. Analysis of the role of ubiquitin-interacting motifs in ubiquitin binding and ubiquitylation. J. Biol. Chem. 279, 33528–33537 (2004).
Oldham, C. E., Mohney, R. P., Miller, S. L., Hanes, R. N. & O'Bryan, J. P. The ubiquitin-interacting motifs target the endocytic adaptor protein epsin for ubiquitination. Curr. Biol. 12, 1112–1116 (2002).
Polo, S. et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416, 451–455 (2002).
Hoeller, D. et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nature Cell Biol. 8, 163–169 (2006). This study shows that the monoubiquitylation of ubiquitin-binding proteins induces intramolecular ubiquitin–UBD interactions that cause the auto-inhibition of UBD-containing proteins.
Broughton, B. C. et al. Molecular analysis of mutations in DNA polymerase eta in xeroderma pigmentosum-variant patients. Proc. Natl Acad. Sci. USA 99, 815–820 (2002).
Kannouche, P. et al. Domain structure, localization, and function of DNA polymerase eta, defective in xeroderma pigmentosum variant cells. Genes Dev. 15, 158–172 (2001).
Brusky, J., Zhu, Y. & Xiao, W. UBC13, a DNA-damage-inducible gene, is a member of the error-free postreplication repair pathway in Saccharomyces cerevisiae. Curr. Genet. 37, 168–174 (2000).
Hofmann, R. M. & Pickart, C. M. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, 645–653 (1999).
Chiu, R. K. et al. Lysine 63-polyubiquitination guards against translesion synthesis-induced mutations. PLoS Genet. 2, e116 (2006).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–133 (2005).
Pfander, B., Moldovan, G. L., Sacher, M., Hoege, C. & Jentsch, S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436, 428–433 (2005). References 84 and 85 provide evidence for a non-competitive but cooperative mode of SUMO and ubiquitin cross-talk in the regulation of DNA synthesis that involves the recruitment of SRS2 to sumoylated PCNA.
Ulrich, H. D., Vogel, S. & Davies, A. A. SUMO keeps a check on recombination during DNA replication. Cell Cycle 4, 1699–1702 (2005).
Melchior, F. SUMO--nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16, 591–626 (2000).
Moon, R. T., Kohn, A. D., De Ferrari, G. V. & Kaykas, A. WNT and β-catenin signalling: diseases and therapies. Nature Rev. Genet. 5, 691–701 (2004).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Willert, K. & Jones, K. A. Wnt signaling: is the party in the nucleus? Genes Dev. 20, 1394–1404 (2006).
Bauer, A. et al. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. EMBO J. 19, 6121–6130 (2000).
Kim, J. H. et al. Roles of sumoylation of a reptin chromatin-remodelling complex in cancer metastasis. Nature Cell Biol. 8, 631–639 (2006). In this report the authors identify specific de-sumoylation enzymes in a purified reptin chromatin-remodelling complex and correlate the sumoylation status of reptin with the invasiveness of cancer cells.
Pitha-Rowe, I. et al. Microarray analyses uncover UBE1L as a candidate target gene for lung cancer chemoprevention. Cancer Res. 64, 8109–8115 (2004).
Pitha-Rowe, I., Hassel, B. A. & Dmitrovsky, E. Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia. J. Biol. Chem. 279, 18178–18187 (2004).
Kalantry, S. et al. Gene rearrangements in the molecular pathogenesis of acute promyelocytic leukemia. J. Cell Physiol. 173, 288–296 (1997).
Pitha-Rowe, I., Petty, W. J., Kitareewan, S. & Dmitrovsky, E. Retinoid target genes in acute promyelocytic leukemia. Leukemia 17, 1723–1730 (2003).
Adams, J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5, 417–421 (2004).
Yang, Y. et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7, 547–559 (2005). This is the first report about the development of inhibitors that target the active site of a RING type E3. The identified compound, HLI98, shows specificity for MDM2 and enabless the activation of p53-dependent transcription in cells.
Buolamwini, J. K. et al. Small molecule antagonists of the MDM2 oncoprotein as anticancer agents. Curr. Cancer Drug Targets 5, 57–68 (2005).
Issaeva, N. et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nature Med. 10, 1321–1328 (2004). The authors identify RITA as a very potent inhibitor of p53 degradation. RITA induced p53-dependent apoptosis in various tumour cells, and showed significant anti-tumour effects in vivo.
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004). In this paper the authors characterize small-molecule inhibitors, called Nutlins, that activate the p53 pathway in cancer cells by binding MDM2 in the p53-binding pocket, thereby preventing p53 ubiquitylation and degradation.
Rubinstein, L. V. et al. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J. Natl Cancer Inst. 82, 1113–1118 (1990).
Krajewski, M., Ozdowy, P., D'Silva, L., Rothweiler, U. & Holak, T. A. NMR indicates that the small molecule RITA does not block p53-MDM2 binding in vitro. Nature Med. 11, 1135–1136; author reply 1136–1137 (2005).
Nalepa, G., Rolfe, M. & Harper, J. W. Drug discovery in the ubiquitin-proteasome system. Nature Rev. Drug Discov. 5, 596–613 (2006).
Mo, Y. Y. & Moschos, S. J. Targeting Ubc9 for cancer therapy. Expert Opin. Ther. Targets 9, 1203–1216 (2005).
Mo, Y. Y., Yu, Y., Theodosiou, E., Rachel Ee, P. L. & Beck, W. T. A role for Ubc9 in tumorigenesis. Oncogene 24, 2677–2683 (2005).
Li, L. et al. A small molecule Smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science 305, 1471–1474 (2004).
Oltersdorf, T. et al. An inhibitor of BCL2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005). By combining nuclear-magnetic-resonance-based screening, parallel synthesis and structure-based design the authors produce potent inhibitors of BCL2 family proteins that target protein–protein interfaces.
Arkin, M. R. & Wells, J. A. Small-molecule inhibitors of protein-protein interactions: progressing towards the dream. Nature Rev. Drug Discov. 3, 301–317 (2004).
Seeler, J. S. & Dejean, A. Nuclear and unclear functions of SUMO. Nature Rev. Mol. Cell Biol. 4, 690–699 (2003).
Pickart, C. M. & Eddins, M. J. Ubiquitin: structures, functions, mechanisms. Biochim. Biophys. Acta 1695, 55–72 (2004).
Oved, S. et al. Conjugation to Nedd8 instigates ubiquitylation and down-regulation of activated receptor tyrosine kinases. J. Biol. Chem. 281, 21640–21651 (2006).
Cadwell, K. & Coscoy, L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309, 127–130 (2005).
Kirkpatrick, D. S. et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biol. 8, 700–710 (2006).
Hipp, M. S., Kalveram, B., Raasi, S., Groettrup, M. & Schmidtke, G. FAT10, a ubiquitin-independent signal for proteasomal degradation. Mol. Cell Biol. 25, 3483–3491 (2005).
Katagiri, Y., Hozumi, Y. & Kondo, S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J. Dermatol. Sci. 42, 215–224 (2006).
Lim, M. S. et al. Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood 100, 2950–2956 (2002).
Wasch, R. & Engelbert, D. Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene 24, 1–10 (2005).
Meetei, A. R. et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nature Genet. 35, 165–170 (2003).
Hu, S. et al. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J. Clin. Invest. 116, 174–181 (2006).
Casas, S. et al. Aberrant expression of HOXA9, DEK, CBL and CSF1R in acute myeloid leukemia. Leuk. Lymphoma 44, 1935–1941 (2003).
Veltman, I. M. et al. Fusion of the SUMO/Sentrin-specific protease 1 gene SENP1 and the embryonic polarity-related mesoderm development gene MESDC2 in a patient with an infantile teratoma and a constitutional t(12;15)(q13;q25). Hum. Mol.Genet. 14, 1955–1963 (2005).
Jacques, C. et al. Two-step differential expression analysis reveals a new set of genes involved in thyroid oncocytic tumors. J. Clin. Endocrinol. Metab. 90, 2314–2320 (2005).
Ledl, A., Schmidt, D. & Muller, S. Viral oncoproteins E1A and E7 and cellular LxCxE proteins repress SUMO modification of the retinoblastoma tumor suppressor. Oncogene 24, 3810–3818 (2005).
Rizos, H., Woodruff, S. & Kefford, R. F. p14ARF interacts with the SUMO-conjugating enzyme Ubc9 and promotes the sumoylation of its binding partners. Cell Cycle 4, 597–603 (2005).
Wang, L. & Banerjee, S. Differential PIAS3 expression in human malignancy. Oncol. Rep. 11, 1319–1324 (2004).
Ueda, M. et al. DNA microarray analysis of stage progression mechanism in myelodysplastic syndrome. Br. J. Haematol. 123, 288–296 (2003).
Villalva, C. et al. Isolation of differentially expressed genes in NPM-ALK-positive anaplastic large cell lymphoma. Br. J. Haematol. 118, 791–798 (2002).
Lee, K. Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts. J. Vet. Sci. 5, 325–330 (2004).
Meehan, K. L., Holland, J. W. & Dawkins, H. J. Proteomic analysis of normal and malignant prostate tissue to identify novel proteins lost in cancer. Prostate 50, 54–63 (2002).
Lee, C. G. et al. Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other gastrointestinal and gynecological cancers. Oncogene 22, 2592–2603 (2003).
Desai, S. D. et al. Elevated expression of ISG15 in tumor cells interferes with the ubiquitin/26S proteasome pathway. Cancer Res. 66, 921–928 (2006).
Kok, K. et al. A gene in the chromosomal region 3p21 with greatly reduced expression in lung cancer is similar to the gene for ubiquitin-activating enzyme. Proc. Natl Acad. Sci. USA 90, 6071–6075 (1993).
Padovan, E. et al. Interferon stimulated gene 15 constitutively produced by melanoma cells induces e-cadherin expression on human dendritic cells. Cancer Res. 62, 3453–3458 (2002).
Ruckes, T., Saul, D., Van Snick, J., Hermine, O. & Grassmann, R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 98, 1150–1159 (2001).
Andersen, J. B. et al. Stage-associated overexpression of the ubiquitin-like protein, ISG15, in bladder cancer. Br. J. Cancer 94, 1465–1471 (2006).
Liu, P. P. et al. Identification of the chimeric protein product of the CBFB-MYH11 fusion gene in inv(16) leukemia cells. Genes Chromosomes Cancer 16, 77–87 (1996).
Tokarz, S. et al. The ISG15 isopeptidase UBP43 is regulated by proteolysis via the SCFSkp2 ubiquitin ligase. J. Biol.Chem. 279, 46424–46430 (2004).
Acknowledgements
We apologize to investigators whose important contributions were not included in this review due to space limitations. We would like to thank D. Vucic and members of the Dikic laboratory for critical reading of the manuscript. C.M.H. is fellow of the Hessian ministry of arts and sciences. I.D. gratefully acknowledges support from the Deutsche Forschungsgemeinschaft, the German–Israeli Foundation and the Boehringer Ingelheim Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Ubl
-
Ubiquitin-like proteins are small proteins that have significant sequence and structural similarity to ubiquitin and are conjugated in a similar way.
- Small ubiquitin-related modifier
-
SUMO belongs to the family of ubiquitin-like proteins. Three ubiquitously expressed paralogues are known. SUMO2 and SUMO3 are highly similar to each other (95%), whereas SUMO1 shows about 50% sequence similarity with SUMO2.
- NEDD8
-
This protein belongs to the family of ubiquitin-like proteins and modifies specific ubiquitin E3 ligases to influence their catalytic ubiquitylation activity.
- Interferon-stimulated gene 15
-
This protein consists of two ubiquitin-like folds, and is conjugated to substrates after interferon 1 stimulation. ISG15 is important in the regulation of the immune response.
- FAT10
-
A ubiquitin-like protein that constists of two ubiquitin-like folds and is conjugated to substrates after interferon-γ and tumour-necrosis factor-α induction. Its expression has been shown to cause apoptosis.
- 26S proteasome
-
A large 2.5 MDa multisubunit protein that is the main proteolytic system in eukaryotic cells. It degrades polyubiquitylated proteins.
- UBDs
-
Ubiquitin binding domains bind to ubiquitin and have crucial roles in the recognition of modified proteins and the translation of encoded information into the proper cellular response.
- SKP1–CUL1–F-box ligase complexes
-
An E3 ligase protein complex that consists of three invariable components: RBX1 (RING-finger protein), CUL1 (scaffold protein) and SKP1 (adaptor protein), and one variable component, the F-box protein.
- Nuclear factor κB
-
A transcription factor that consists of either a homo- or a heterodimer of proteins such as NFκB1, RelA, NFκB2 and RelB.
- Ataxia telangiectasia mutated
-
ATM is a crucial signal-transducing kinase for mediating certain forms of DNA damage such as double-strand breaks. The activation of many proteins has been shown to be ATM-dependent. These include CHK1 and CHK2 kinases, BRCA1, NBS1, and p53, among others.
- UBM and UBZ
-
Ubiquitin-binding motif (UBM) and ubiquitin-binding zinc finger (UBZ) were recently discovered to be involved in the TLS response. UBMs do not bind to the hydrophobic Ile44 patch of ubiquitin.
- Y-family polymerases
-
These are translesion synthesis (TLS) polymerases of low fidelity, such as Polι, Polη, Polκ and REV1, which are involved in the error-prone DNA-repair pathway.
- Wnt
-
The family of secreted cysteine-rich glycoproteins that act as short-range ligands to locally activate receptor-mediated pathways.
- KAI1
-
A member of the tetraspanin family that is capable of inhibiting the progression of tumour metastasis without affecting primary tumorigenicity.
- All trans retinoic acid
-
A form of vitamin A used as a drug for the treatment of acute promyelocytic leukaemia.
Rights and permissions
About this article
Cite this article
Hoeller, D., Hecker, CM. & Dikic, I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer 6, 776–788 (2006). https://doi.org/10.1038/nrc1994
Issue Date:
DOI: https://doi.org/10.1038/nrc1994
This article is cited by
-
The E3 ligase NEURL3 suppresses epithelial-mesenchymal transition and metastasis in nasopharyngeal carcinoma by promoting vimentin degradation
Journal of Experimental & Clinical Cancer Research (2024)
-
MKRN1 promotes colorectal cancer metastasis by activating the TGF-β signalling pathway through SNIP1 protein degradation
Journal of Experimental & Clinical Cancer Research (2023)
-
ISG15 and ISGylation modulates cancer stem cell-like characteristics in promoting tumor growth of anaplastic thyroid carcinoma
Journal of Experimental & Clinical Cancer Research (2023)
-
UBE4B interacts with the ITCH E3 ubiquitin ligase to induce Ku70 and c-FLIPL polyubiquitination and enhanced neuroblastoma apoptosis
Cell Death & Disease (2023)
-
TRIM21 inhibits irradiation-induced mitochondrial DNA release and impairs antitumour immunity in nasopharyngeal carcinoma tumour models
Nature Communications (2023)