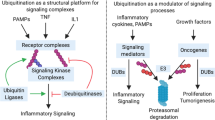
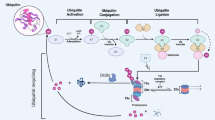
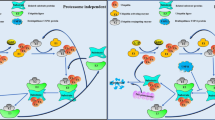
Abstract
Ubiquitination is an essential regulator of most, if not all, signalling pathways, and defects in cellular signalling are central to cancer initiation, progression and, eventually, metastasis. The attachment of ubiquitin signals by E3 ubiquitin ligases is directly opposed by the action of approximately 100 deubiquitinating enzymes (DUBs) in humans. Together, DUBs and E3 ligases coordinate ubiquitin signalling by providing selectivity for different substrates and/or ubiquitin signals. The balance between ubiquitination and deubiquitination is exquisitely controlled to ensure properly coordinated proteostasis and response to cellular stimuli and stressors. Not surprisingly, then, DUBs have been associated with all hallmarks of cancer. These relationships are often complex and multifaceted, highlighted by the implication of multiple DUBs in certain hallmarks and by the impact of individual DUBs on multiple cancer-associated pathways, sometimes with contrasting cancer-promoting and cancer-inhibiting activities, depending on context and tumour type. Although it is still understudied, the ever-growing knowledge of DUB function in cancer physiology will eventually identify DUBs that warrant specific inhibition or activation, both of which are now feasible. An integrated appreciation of the physiological consequences of DUB modulation in relevant cancer models will eventually lead to the identification of patient populations that will most likely benefit from DUB-targeted therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Swatek, K. N. & Komander, D. Ubiquitin modifications. Cell Res. 26, 399–422 (2016).
Komander, D. & Rape, M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012).
Dikic, I. & Schulman, B. A. An expanded lexicon for the ubiquitin code. Nat. Rev. Mol. Cell Biol. 24, 273–287 (2022).
Lacoursiere, R. E., Hadi, D. & Shaw, G. S. Acetylation, phosphorylation, ubiquitination (oh my!): following post-translational modifications on the ubiquitin road. Biomolecules 12, 467 (2022).
Zheng, N. & Shabek, N. Ubiquitin ligases: structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017).
Husnjak, K. & Dikic, I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu. Rev. Biochem. 81, 291–322 (2012).
Clague, M. J. & Urbe, S. Ubiquitin: same molecule, different degradation pathways. Cell 143, 682–685 (2010).
Meyer, H., Bug, M. & Bremer, S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 14, 117–123 (2012).
Hurley, J. H. The ESCRT complexes. Crit. Rev. Biochem. Mol. Biol. 45, 463–487 (2010).
Dikic, I. & Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 19, 349–364 (2018).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Ea, C. K., Deng, L., Xia, Z. P., Pineda, G. & Chen, Z. J. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol. Cell 22, 245–257 (2006).
Wu, C. J., Conze, D. B., Li, T., Srinivasula, S. M. & Ashwell, J. D. Sensing of Lys63-linked polyubiquitination by NEMO is a key event in NF-κB activation [corrected]. Nat. Cell Biol. 8, 398–406 (2006).
Hrdinka, M. & Gyrd-Hansen, M. The Met1-linked ubiquitin machinery: emerging themes of (de)regulation. Mol. Cell 68, 265–280 (2017).
Harper, J. W., Ordureau, A. & Heo, J. M. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 19, 93–108 (2018).
Clague, M. J., Urbe, S. & Komander, D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 20, 338–352 (2019). This work presents a comprehensive overview of DUB specificity and how this influences cell biology.
Mevissen, T. E. T. & Komander, D. Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159–192 (2017). This work presents a comprehensive overview of DUB specificity and mechanism of action.
Ye, Y., Scheel, H., Hofmann, K. & Komander, D. Dissection of USP catalytic domains reveals five common insertion points. Mol. Biosyst. 5, 1797–1808 (2009).
Kumari, N. et al. The roles of ubiquitin modifying enzymes in neoplastic disease. Biochim. Biophys. Acta Rev. Cancer 1868, 456–483 (2017).
Sahtoe, D. D. & Sixma, T. K. Layers of DUB regulation. Trends Biochem. Sci. 40, 456–467 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Sacco, J. J., Coulson, J. M., Clague, M. J. & Urbe, S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life 62, 140–157 (2010).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000). This seminal paper discusses the cellular pathways that drive oncogenesis.
Keusekotten, K. et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell 153, 1312–1326 (2013).
Nakagawa, T. et al. Deubiquitylation of histone H2A activates transcriptional initiation via trans-histone cross-talk with H3K4 di- and trimethylation. Genes. Dev. 22, 37–49 (2008).
Glinsky, G. V. Genomic models of metastatic cancer: functional analysis of death-from-cancer signature genes reveals aneuploid, anoikis-resistant, metastasis-enabling phenotype with altered cell cycle control and activated Polycomb group (PcG) protein chromatin silencing pathway. Cell Cycle 5, 1208–1216 (2006).
Zhang, X. Y. et al. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol. Cell 29, 102–111 (2008). This paper links the USP22 DUB to the SAGA transcriptional regulator complex that probably underpins its ‘death-from-cancer’ gene status.
Alexandrov, L. B. et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Compagno, M. et al. Mutations of multiple genes cause deregulation of NF-κB in diffuse large B-cell lymphoma. Nature 459, 717–721 (2009).
Schmitz, R. et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J. Exp. Med. 206, 981–989 (2009).
Testa, J. R. et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 43, 1022–1025 (2011).
Popova, T. et al. Germline BAP1 mutations predispose to renal cell carcinomas. Am. J. Hum. Genet. 92, 974–980 (2013).
Tesch, M. E. et al. Concurrent germline and somatic pathogenic BAP1 variants in a patient with metastatic bladder cancer. NPJ Genom. Med. 5, 12 (2020).
Masclef, L. et al. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 28, 606–625 (2021).
Harbour, J. W. et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 330, 1410–1413 (2010). This study identifies mutations in BAP1 that underlie tumour development.
Bignell, G. R. et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat. Genet. 25, 160–165 (2000). This study identifies CYLD mutation as the cause of a familial tumour predisposition syndrome.
Wang, Y. & Wang, F. Post-translational modifications of deubiquitinating enzymes: expanding the ubiquitin code. Front. Pharmacol. 12, 685011 (2021).
Das, T., Shin, S. C., Song, E. J. & Kim, E. E. Regulation of deubiquitinating enzymes by post-translational modifications. Int. J. Mol. Sci. 21, 4028 (2020).
Sauer, F. et al. Differential oligomerization of the deubiquitinases USP25 and USP28 regulates their activities. Mol. Cell 74, 421–435.e10 (2019).
Ren, X. et al. Ubiquitin-specific protease 28: the decipherment of its dual roles in cancer development. Exp. Hematol. Oncol. 12, 27 (2023).
Oliveira, A. M. & Chou, M. M. The TRE17/USP6 oncogene: a riddle wrapped in a mystery inside an enigma. Front. Biosci. 4, 321–334 (2012).
Matthews, H. K., Bertoli, C. & de Bruin, R. A. M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 23, 74–88 (2022).
Nakayama, K. I. & Nakayama, K. Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer 6, 369–381 (2006).
Ye, Y. & Rape, M. Building ubiquitin chains: E2 enzymes at work. Nat. Rev. Mol. Cell Biol. 10, 755–764 (2009).
Meyer, H. J. & Rape, M. Enhanced protein degradation by branched ubiquitin chains. Cell 157, 910–921 (2014). This paper reports that the APC/C complex catalyses Lys11-linked branched chains that enhance proteasomal degradation of substrates.
Bonacci, T. et al. Cezanne/OTUD7B is a cell cycle-regulated deubiquitinase that antagonizes the degradation of APC/C substrates. EMBO J. 37, e98701 (2018).
Stegmeier, F. et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 446, 876–881 (2007).
Mevissen, T. E. et al. OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154, 169–184 (2013).
Shan, J., Zhao, W. & Gu, W. Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol. Cell 36, 469–476 (2009).
Galarreta, A. et al. USP7 limits CDK1 activity throughout the cell cycle. EMBO J. 40, e99692 (2021).
Pal, A., Young, M. A. & Donato, N. J. Emerging potential of therapeutic targeting of ubiquitin-specific proteases in the treatment of cancer. Cancer Res. 74, 4955–4966 (2014).
Turnbull, A. P. et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature 550, 481–486 (2017). This important paper provides a proof of concept that DUBs are targetable with selective small-molecule inhibitors and identifies specific inhibitors of USP7.
Zhang, Y. et al. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J. Clin. Invest. 122, 4362–4374 (2012).
Park, J., Kwon, M. S., Kim, E. E., Lee, H. & Song, E. J. USP35 regulates mitotic progression by modulating the stability of Aurora B. Nat. Commun. 9, 688 (2018).
van Leuken, R. J., Luna-Vargas, M. P., Sixma, T. K., Wolthuis, R. M. & Medema, R. H. Usp39 is essential for mitotic spindle checkpoint integrity and controls mRNA-levels of aurora B. Cell Cycle 7, 2710–2719 (2008).
Lu, Y. et al. USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol. Cell Biol. 29, 547–558 (2009).
Hu, W. et al. Ubiquitin specific peptidase 19 is a prognostic biomarker and affect the proliferation and migration of clear cell renal cell carcinoma. Oncol. Rep. 43, 1964–1974 (2020).
Faustrup, H., Bekker-Jensen, S., Bartek, J., Lukas, J. & Mailand, N. USP7 counteracts SCFβTrCP- but not APCCdh1-mediated proteolysis of Claspin. J. Cell Biol. 184, 13–19 (2009).
Alonso-de Vega, I., Martin, Y. & Smits, V. A. USP7 controls Chk1 protein stability by direct deubiquitination. Cell Cycle 13, 3921–3926 (2014).
Cheng, Y. C. & Shieh, S. Y. Deubiquitinating enzyme USP3 controls CHK1 chromatin association and activation. Proc. Natl Acad. Sci. USA 115, 5546–5551 (2018).
Bhattacharya, S. & Ghosh, M. K. HAUSP, a novel deubiquitinase for Rb—MDM2 the critical regulator. FEBS J. 281, 3061–3078 (2014).
Li, M., Brooks, C. L., Kon, N. & Gu, W. A dynamic role of HAUSP in the p53–Mdm2 pathway. Mol. Cell 13, 879–886 (2004).
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002). This key paper establishes the USP7–MDM2–p53 connection, one of the main reasons that USP7 is a proposed target in cancer.
Yuan, J., Luo, K., Zhang, L., Cheville, J. C. & Lou, Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell 140, 384–396 (2010). This paper highlights the role of USP10 in dictating p53 localization and stability.
Das, S. K., Lewis, B. A. & Levens, D. MYC: a complex problem. Trends Cell Biol. 33, 235–246 (2023).
Amati, B. & Sanchez-Arevalo Lobo, V. J. MYC degradation: deubiquitinating enzymes enter the dance. Nat. Cell Biol. 9, 729–731 (2007).
Popov, N. et al. The ubiquitin-specific protease USP28 is required for MYC stability. Nat. Cell Biol. 9, 765–774 (2007). This key paper identifies regulation of MYC by USP28.
Sun, X. X. et al. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc. Natl Acad. Sci. USA 112, 3734–3739 (2015).
Xu, Z., Hu, H., Fang, D., Wang, J. & Zhao, K. The deubiquitinase USP38 promotes cell proliferation through stabilizing c-Myc. Int. J. Biochem. Cell Biol. 137, 106023 (2021).
Yada, M. et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 (2004).
Wang, Z., Song, Q., Xue, J., Zhao, Y. & Qin, S. Ubiquitin-specific protease 28 is overexpressed in human glioblastomas and contributes to glioma tumorigenicity by regulating MYC expression. Exp. Biol. Med. 241, 255–264 (2016).
Schulein-Volk, C. et al. Dual regulation of Fbw7 function and oncogenic transformation by Usp28. Cell Rep. 9, 1099–1109 (2014).
Qin, T. et al. Abnormally elevated USP37 expression in breast cancer stem cells regulates stemness, epithelial–mesenchymal transition and cisplatin sensitivity. J. Exp. Clin. Cancer Res. 37, 287 (2018).
Pan, J. et al. USP37 directly deubiquitinates and stabilizes c-Myc in lung cancer. Oncogene 34, 3957–3967 (2015).
Huang, X. et al. Deubiquitinase USP37 is activated by CDK2 to antagonize APC(CDH1) and promote S phase entry. Mol. Cell 42, 511–523 (2011).
Fang, X. et al. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J. Exp. Med. 214, 245–267 (2017).
Kim, D. et al. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J. Cell Physiol. 232, 3664–3676 (2017).
Schrecengost, R. S. et al. USP22 regulates oncogenic signaling pathways to drive lethal cancer progression. Cancer Res. 74, 272–286 (2014).
Lin, Z. et al. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 5, 1639–1649 (2013).
Tao, L. et al. USP10 as a potential therapeutic target in human cancers. Genes 13, 831 (2022).
Ruiz, E. J. et al. USP28 deletion and small-molecule inhibition destabilizes c-MYC and elicits regression of squamous cell lung carcinoma. eLife 10, e75196 (2021).
Prieto-Garcia, C. et al. Maintaining protein stability of ∆Np63 via USP28 is required by squamous cancer cells. EMBO Mol. Med. 12, e11101 (2020).
Saei, A. et al. Loss of USP28-mediated BRAF degradation drives resistance to RAF cancer therapies. J. Exp. Med. 215, 1913–1928 (2018).
Tavana, O. et al. HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma. Nat. Med. 22, 1180–1186 (2016).
Grunblatt, E. et al. MYCN drives chemoresistance in small cell lung cancer while USP7 inhibition can restore chemosensitivity. Genes. Dev. 34, 1210–1226 (2020).
Fruman, D. A. et al. The PI3K pathway in human disease. Cell 170, 605–635 (2017).
Papa, A. & Pandolfi, P. P. The PTEN–PI3K axis in cancer. Biomolecules 9, 153 (2019).
Gonzalez-Garcia, A., Garrido, A. & Carrera, A. C. Targeting PTEN regulation by post translational modifications. Cancers 14, 5613 (2022).
Song, M. S. et al. The deubiquitinylation and localization of PTEN are regulated by a HAUSP–PML network. Nature 455, 813–817 (2008). This paper reports the regulation of the tumour suppressor PTEN by USP7.
Park, M. K. et al. PTEN self-regulates through USP11 via the PI3K–FOXO pathway to stabilize tumor suppression. Nat. Commun. 10, 636 (2019).
Zhang, J. et al. Deubiquitylation and stabilization of PTEN by USP13. Nat. Cell Biol. 15, 1486–1494 (2013).
Yuan, L. et al. Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat. Cell Biol. 17, 1169–1181 (2015).
Qu, Z., Zhang, R., Su, M. & Liu, W. USP13 serves as a tumor suppressor via the PTEN/AKT pathway in oral squamous cell carcinoma. Cancer Manag. Res. 11, 9175–9183 (2019).
Man, X. et al. USP13 functions as a tumor suppressor by blocking the NF-κB-mediated PTEN downregulation in human bladder cancer. J. Exp. Clin. Cancer Res. 38, 259 (2019).
Kapadia, B. et al. Fatty acid synthase induced S6Kinase facilitates USP11–eIF4B complex formation for sustained oncogenic translation in DLBCL. Nat. Commun. 9, 829 (2018).
Li, Y. et al. USP13 regulates the RAP80–BRCA1 complex dependent DNA damage response. Nat. Commun. 8, 15752 (2017).
Mossmann, D., Park, S. & Hall, M. N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 18, 744–757 (2018).
Cho, J. H. et al. Deubiquitinase OTUD5 is a positive regulator of mTORC1 and mTORC2 signaling pathways. Cell Death Differ. 28, 900–914 (2021).
Kosinsky, R. L. et al. USP22 exerts tumor-suppressive functions in colorectal cancer by decreasing mTOR activity. Cell Death Differ. 27, 1328–1340 (2020).
Wang, D. et al. E3 ligase RNF167 and deubiquitinase STAMBPL1 modulate mTOR and cancer progression. Mol. Cell 82, 770–784.e9 (2022).
Wang, B. et al. TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling. Nature 545, 365–369 (2017).
Bremm, A., Freund, S. M. & Komander, D. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat. Struct. Mol. Biol. 17, 939–947 (2010).
Potu, H. et al. Downregulation of SOX2 by inhibition of Usp9X induces apoptosis in melanoma. Oncotarget 12, 160–172 (2021).
Shay, J. W. & Wright, W. E. Role of telomeres and telomerase in cancer. Semin. Cancer Biol. 21, 349–353 (2011).
Harley, C. B. Telomerase and cancer therapeutics. Nat. Rev. Cancer 8, 167–179 (2008).
Cesare, A. J. & Reddel, R. R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat. Rev. Genet. 11, 319–330 (2010).
Bejarano, L. et al. Safety of whole-body abrogation of the TRF1 shelterin protein in wild-type and cancer-prone mouse models. iScience 19, 572–585 (2019).
Bejarano, L. et al. Inhibition of TRF1 telomere protein impairs tumor initiation and progression in glioblastoma mouse models and patient-derived xenografts. Cancer Cell 32, 590–607.e4 (2017).
Garcia-Beccaria, M. et al. Therapeutic inhibition of TRF1 impairs the growth of p53-deficient K-RasG12V-induced lung cancer by induction of telomeric DNA damage. EMBO Mol. Med. 7, 930–949 (2015).
Atanassov, B. S. et al. Gcn5 and SAGA regulate shelterin protein turnover and telomere maintenance. Mol. Cell 35, 352–364 (2009).
Zemp, I. & Lingner, J. The shelterin component TPP1 is a binding partner and substrate for the deubiquitinating enzyme USP7. J. Biol. Chem. 289, 28595–28606 (2014).
Episkopou, H., Diman, A., Claude, E., Viceconte, N. & Decottignies, A. TSPYL5 depletion induces specific death of ALT cells through USP7-dependent proteasomal degradation of POT1. Mol. Cell 75, 469–482.e6 (2019).
Abercrombie, M. Contact inhibition and malignancy. Nature 281, 259–262 (1979).
Christofori, G. & Semb, H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem. Sci. 24, 73–76 (1999).
Gumbiner, B. M. & Kim, N. G. The Hippo–YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 127, 709–717 (2014).
Gao, Y. et al. Usp10 modulates the Hippo pathway by deubiquitinating and stabilizing the transcriptional coactivator Yorkie. Int. J. Mol. Sci. 20, 6013 (2019).
Sun, X. et al. Usp7 regulates Hippo pathway through deubiquitinating the transcriptional coactivator Yorkie. Nat. Commun. 10, 411 (2019).
Zhu, H. et al. USP10 promotes proliferation of hepatocellular carcinoma by deubiquitinating and stabilizing YAP/TAZ. Cancer Res. 80, 2204–2216 (2020).
Pan, B. et al. USP47-mediated deubiquitination and stabilization of YAP contributes to the progression of colorectal cancer. Protein Cell 11, 138–143 (2020).
Yao, F. et al. SKP2- and OTUD1-regulated non-proteolytic ubiquitination of YAP promotes YAP nuclear localization and activity. Nat. Commun. 9, 2269 (2018).
Schenk, R. L., Strasser, A. & Dewson, G. BCL-2: long and winding path from discovery to therapeutic target. Biochem. Biophys. Res. Commun. 482, 459–469 (2017).
Roberts, J. Z., Crawford, N. & Longley, D. B. The role of ubiquitination in apoptosis and necroptosis. Cell Death Differ. 29, 272–284 (2021).
Lork, M., Verhelst, K. & Beyaert, R. CYLD, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: so similar, yet so different. Cell Death Differ. 24, 1172–1183 (2017).
Czabotar, P. E., Lessene, G., Strasser, A. & Adams, J. M. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014).
Roberts, A. W. et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 374, 311–322 (2016).
Djajawi, T. M. et al. MARCH5 requires MTCH2 to coordinate proteasomal turnover of the MCL1:NOXA complex. Cell Death Differ. 27, 2484–2499 (2020).
Zhong, Q., Gao, W., Du, F. & Wang, X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121, 1085–1095 (2005).
Carroll, R. G., Hollville, E. & Martin, S. J. Parkin sensitizes toward apoptosis induced by mitochondrial depolarization through promoting degradation of Mcl-1. Cell Rep. 9, 1538–1553 (2014).
Schwickart, M. et al. Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463, 103–107 (2010).
Zhang, S. et al. Deubiquitinase USP13 dictates MCL1 stability and sensitivity to BH3 mimetic inhibitors. Nat. Commun. 9, 215 (2018).
Wu, X. et al. MGMT-activated DUB3 stabilizes MCL1 and drives chemoresistance in ovarian cancer. Proc. Natl Acad. Sci. USA 116, 2961–2966 (2019).
Trivigno, D., Essmann, F., Huber, S. M. & Rudner, J. Deubiquitinase USP9x confers radioresistance through stabilization of Mcl-1. Neoplasia 14, 893–904 (2012).
Wu, L. et al. The deubiquitinating enzyme OTUD1 antagonizes BH3-mimetic inhibitor induced cell death through regulating the stability of the MCL1 protein. Cancer Cell Int. 19, 222 (2019).
Weber, A. et al. The deubiquitinase Usp27x stabilizes the BH3-only protein Bim and enhances apoptosis. EMBO Rep. 17, 724–738 (2016).
Johnson, B. N., Berger, A. K., Cortese, G. P. & Lavoie, M. J. The ubiquitin E3 ligase parkin regulates the proapoptotic function of Bax. Proc. Natl Acad. Sci. USA 109, 6283–6288 (2012).
Bernardini, J. P. et al. Parkin inhibits BAK and BAX apoptotic function by distinct mechanisms during mitophagy. EMBO J. 38, e99916 (2019).
Benard, G. et al. IBRDC2, an IBR-type E3 ubiquitin ligase, is a regulatory factor for Bax and apoptosis activation. EMBO J. 29, 1458–1471 (2010).
Bingol, B. et al. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature 510, 370–375 (2014).
Liang, J. R. et al. USP30 deubiquitylates mitochondrial Parkin substrates and restricts apoptotic cell death. EMBO Rep. 16, 618–627 (2015).
Yan, D. et al. Regulation of Bax-dependent apoptosis by mitochondrial deubiquitinase USP30. Cell Death Discov. 7, 211 (2021).
Zhou, Z. et al. Regulation of XIAP turnover reveals a role for USP11 in promotion of tumorigenesis. EBioMedicine 15, 48–61 (2017).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Tang, Z. et al. Deubiquitinase USP35 modulates ferroptosis in lung cancer via targeting ferroportin. Clin. Transl. Med. 11, e390 (2021).
Liu, T., Jiang, L., Tavana, O. & Gu, W. The deubiquitylase OTUB1 mediates ferroptosis via stabilization of SLC7A11. Cancer Res. 79, 1913–1924 (2019).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Wang, Y. et al. The deubiquitinase USP22 regulates PD-L1 degradation in human cancer cells. Cell Commun. Signal. 18, 112 (2020).
Lim, S. O. et al. Deubiquitination and stabilization of PD-L1 by CSN5. Cancer Cell 30, 925–939 (2016).
Zhu, D. et al. Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ. 28, 1773–1789 (2021).
Pan, J. et al. USP5 facilitates non-small cell lung cancer progression through stabilization of PD-L1. Cell death Dis. 12, 1051 (2021).
Jingjing, W. et al. Deubiquitination and stabilization of programmed cell death ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral squamous cell carcinoma. Cancer Med. 7, 4004–4011 (2018).
Zhu, D. et al. Deubiquitinating enzyme OTUB1 promotes cancer cell immunosuppression via preventing ER-associated degradation of immune checkpoint protein PD-L1. Cell Death Differ. 28, 1773–1789 (2020).
Wang, B., Cai, W., Ai, D., Zhang, X. & Yao, L. The role of deubiquitinases in vascular diseases. J. Cardiovasc. Transl. Res. 13, 131–141 (2020).
Hashimoto, T. & Shibasaki, F. Hypoxia-inducible factor as an angiogenic master switch. Front. Pediatr. 3, 33 (2015).
Li, Z., Wang, D., Messing, E. M. & Wu, G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1α. EMBO Rep. 6, 373–378 (2005).
Wu, H. T. et al. K63-polyubiquitinated HAUSP deubiquitinates HIF-1α and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat. Commun. 7, 13644 (2016).
Altun, M. et al. Ubiquitin-specific protease 19 (USP19) regulates hypoxia-inducible factor 1α (HIF-1α) during hypoxia. J. Biol. Chem. 287, 1962–1969 (2012).
Flugel, D., Gorlach, A. & Kietzmann, T. GSK-3β regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1α. Blood 119, 1292–1301 (2012).
Luong le, A. et al. Cezanne regulates inflammatory responses to hypoxia in endothelial cells by targeting TRAF6 for deubiquitination. Circ. Res. 112, 1583–1591 (2013).
Moniz, S. et al. Cezanne regulates E2F1-dependent HIF2α expression. J. Cell Sci. 128, 3082–3093 (2015).
Mader, J. et al. Oxygen-dependent asparagine hydroxylation of the ubiquitin-associated (UBA) domain in Cezanne regulates ubiquitin binding. J. Biol. Chem. 295, 2160–2174 (2020).
Bremm, A., Moniz, S., Mader, J., Rocha, S. & Komander, D. Cezanne (OTUD7B) regulates HIF-1α homeostasis in a proteasome-independent manner. EMBO Rep. 15, 1268–1277 (2014).
Rivkin, E. et al. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature 498, 318–324 (2013).
Fu, Y. et al. OTULIN allies with LUBAC to govern angiogenesis by editing ALK1 linear polyubiquitin. Mol. Cell 81, 3187–3204.e7 (2021). This paper reports the mechanism behind the physiological data on OTULIN presented by Rivkin et al. (2013) implicating Met1-linked chains in ALK kinase regulation.
Damgaard, R. B. et al. OTULIN protects the liver against cell death, inflammation, fibrosis, and cancer. Cell Death Differ. 27, 1457–1474 (2020).
Damgaard, R. B. et al. OTULIN deficiency in ORAS causes cell type-specific LUBAC degradation, dysregulated TNF signalling and cell death. EMBO Mol. Med. 11, e9324 (2019).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells. Trends Biochem. Sci. 41, 211–218 (2016).
Harris, I. S. et al. Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab. 29, 1166–1181.e6 (2019).
Kapuria, V. et al. Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 70, 9265–9276 (2010).
Strickson, S. et al. The anti-inflammatory drug BAY 11-7082 suppresses the MyD88-dependent signalling network by targeting the ubiquitin system. Biochem. J. 451, 427–437 (2013).
Wang, Q., Li, L. & Ye, Y. Inhibition of p97-dependent protein degradation by Eeyarestatin I. J. Biol. Chem. 283, 7445–7454 (2008).
Altun, M. et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem. Biol. 18, 1401–1412 (2011).
Lee, J., Rhee, M. H., Kim, E. & Cho, J. Y. BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediators Inflamm. 2012, 416036 (2012).
Fontan, L. et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell 22, 812–824 (2012).
Jiang, L. et al. Ubiquitin-specific peptidase 7 (USP7)-mediated deubiquitination of the histone deacetylase SIRT7 regulates gluconeogenesis. J. Biol. Chem. 292, 13296–13311 (2017).
Barber, M. F. et al. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature 487, 114–118 (2012).
Hall, J. A., Tabata, M., Rodgers, J. T. & Puigserver, P. USP7 attenuates hepatic gluconeogenesis through modulation of FoxO1 gene promoter occupancy. Mol. Endocrinol. 28, 912–924 (2014).
Han, C. et al. Amplification of USP13 drives ovarian cancer metabolism. Nat. Commun. 7, 13525 (2016).
Yu, S., Zang, W., Qiu, Y., Liao, L. & Zheng, X. Deubiquitinase OTUB2 exacerbates the progression of colorectal cancer by promoting PKM2 activity and glycolysis. Oncogene 41, 46–56 (2022).
Kumar, A. et al. Disruption of the autoinhibited state primes the E3 ligase parkin for activation and catalysis. EMBO J. 34, 2506–2521 (2015).
Hashimoto, M. et al. Inhibition of ubiquitin-specific protease 2 causes accumulation of reactive oxygen species, mitochondria dysfunction, and intracellular ATP decrement in C2C12 myoblasts. Physiol. Rep. 7, e14193 (2019).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J. P. EMT: 2016. Cell 166, 21–45 (2016).
Boumahdi, S. & de Sauvage, F. J. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug. Discov. 19, 39–56 (2020).
Wu, Y. et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat. Commun. 8, 14228 (2017).
Liu, T. et al. CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat. Commun. 8, 13923 (2017).
He, J. et al. Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death Dis. 10, 285 (2019).
Zhu, Y. et al. Trabid inhibits hepatocellular carcinoma growth and metastasis by cleaving RNF8-induced K63 ubiquitination of Twist1. Cell Death Differ. 26, 306–320 (2019).
Massague, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Huang, C. Y. et al. Recent progress in TGF-β inhibitors for cancer therapy. Biomed. Pharmacother. 134, 111046 (2021).
Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F. & Wrana, J. L. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 5, 410–421 (2003).
Kavsak, P. et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol. Cell 6, 1365–1375 (2000).
Zhang, L. et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat. Cell Biol. 14, 717–726 (2012).
Liu, S., de Boeck, M., van Dam, H. & Ten Dijke, P. Regulation of the TGF-β pathway by deubiquitinases in cancer. Int. J. Biochem. Cell Biol. 76, 135–145 (2016).
Eichhorn, P. J. et al. USP15 stabilizes TGF-β receptor I and promotes oncogenesis through the activation of TGF-β signaling in glioblastoma. Nat. Med. 18, 429–435 (2012). This paper reports that USP15 regulates TGFβ signalling and identifies it as a new target for the treatment of glioblastoma.
Al-Salihi, M. A., Herhaus, L., Macartney, T. & Sapkota, G. P. USP11 augments TGFβ signalling by deubiquitylating ALK5. Open. Biol. 2, 120063 (2012).
Iyengar, P. V. et al. USP15 regulates SMURF2 kinetics through C-lobe mediated deubiquitination. Sci. Rep. 5, 14733 (2015).
Elliott, P. R. et al. Structural variability of the ubiquitin specific protease DUSP–UBL double domains. FEBS Lett. 585, 3385–3390 (2011).
Zhang, Z. et al. Breast cancer metastasis suppressor OTUD1 deubiquitinates SMAD7. Nat. Commun. 8, 2116 (2017).
Mevissen, T. E. T. et al. Molecular basis of Lys11-polyubiquitin specificity in the deubiquitinase Cezanne. Nature 538, 402–405 (2016).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
van Loo, G. & Bertrand, M. J. M. Death by TNF: a road to inflammation. Nat. Rev. Immunol. 23, 289–303 (2023).
Peltzer, N. & Walczak, H. Cell death and inflammation — a vital but dangerous liaison. Trends Immunol. 40, 387–402 (2019).
Shibata, Y. & Komander, D. LUBAC. Curr. Biol. 32, R506–R508 (2022).
Kovalenko, A. et al. The tumour suppressor CYLD negatively regulates NF-κB signalling by deubiquitination. Nature 424, 801–805 (2003).
Trompouki, E. et al. CYLD is a deubiquitinating enzyme that negatively regulates NF-κB activation by TNFR family members. Nature 424, 793–796 (2003).
Brummelkamp, T. et al. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424, 797–801 (2003). This key paper, along with Kovalenko (2003) and Trompouki et al. (2003), characterized the molecular mechanism of CYLD’s regulation of NF-kB signalling that underpins CYLD’s tumour suppressor function.
Enesa, K. et al. NF-κB suppression by the deubiquitinating enzyme Cezanne: a novel negative feedback loop in pro-inflammatory signaling. J. Biol. Chem. 283, 7036–7045 (2008).
Schlicher, L. et al. SPATA2 promotes CYLD activity and regulates TNF-induced NF-κB signaling and cell death. EMBO Rep. 17, 1485–1497 (2016).
Wagner, S. A., Satpathy, S., Beli, P. & Choudhary, C. SPATA2 links CYLD to the TNF-α receptor signaling complex and modulates the receptor signaling outcomes. EMBO J. 35, 1868–1884 (2016).
Elliott, P. R. et al. Regulation of CYLD activity and specificity by phosphorylation and ubiquitin-binding CAP-Gly domains. Cell Rep. 37, 109777 (2021).
Massoumi, R., Chmielarska, K., Hennecke, K., Pfeifer, A. & Fassler, R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-κB signaling. Cell 125, 665–677 (2006).
Stegmeier, F. et al. The tumor suppressor CYLD regulates entry into mitosis. Proc. Natl Acad. Sci. USA 104, 8869–8874 (2007).
Fernandez-Majada, V. et al. The tumour suppressor CYLD regulates the p53 DNA damage response. Nat. Commun. 7, 12508 (2016).
Evans, P. C. et al. Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. Biochem. J. 378, 727–734 (2004).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
De, A., Dainichi, T., Rathinam, C. V. & Ghosh, S. The deubiquitinase activity of A20 is dispensable for NF-κB signaling. EMBO Rep. 15, 775–783 (2014).
Honma, K. et al. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 114, 2467–2475 (2009).
Damgaard, R. B. et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell 166, 1215–1230.e20 (2016). This key paper identifies the Met1-specific DUB OTULIN as an essential negative regulator of TNF signalling and inflammation.
Stangl, A. et al. Regulation of the endosomal SNX27-retromer by OTULIN. Nat. Commun. 10, 4320 (2019).
Fiil, B. K. et al. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol. Cell 50, 818–830 (2013).
Verboom, L. et al. OTULIN prevents liver inflammation and hepatocellular carcinoma by inhibiting FADD- and RIPK1 kinase-mediated hepatocyte apoptosis. Cell Rep. 30, 2237–2247.e36 (2020).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
McArthur, K. et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 359, 883 (2018).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011). This study identifies a key role for mitochondria as a platform for NLRP3 inflammatory signalling.
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Riley, J. S. et al. Mitochondrial inner membrane permeabilisation enables mtDNA release during apoptosis. EMBO J. 37, e99238 (2018).
Minton, K. Inflammasome: anti-inflammatory effect of mitophagy. Nat. Rev. Immunol. 16, 206 (2016).
Narendra, D., Tanaka, A., Suen, D. F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Pickrell, A. M. & Youle, R. J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 85, 257–273 (2015).
Bernardini, J. P., Lazarou, M. & Dewson, G. Parkin and mitophagy in cancer. Oncogene 36, 1315–1327 (2017).
Veeriah, S. et al. Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nat. Genet. 42, 77–82 (2010).
Gong, Y. et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat. Genet. 46, 588–594 (2014).
Gupta, A. et al. PARK2 depletion connects energy and oxidative stress to PI3K/Akt activation via PTEN S-nitrosylation. Mol. Cell 65, 999–1013.e7 (2017).
Fujiwara, M. et al. Parkin as a tumor suppressor gene for hepatocellular carcinoma. Oncogene 27, 6002–6011 (2008).
Cunningham, C. N. et al. USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nat. Cell Biol. 17, 160–169 (2015).
Cornelissen, T. et al. The deubiquitinase USP15 antagonizes Parkin-mediated mitochondrial ubiquitination and mitophagy. Hum. Mol. Genet. 23, 5227–5242 (2014).
Gu, L. et al. The IKKβ–USP30–ACLY axis controls lipogenesis and tumorigenesis. Hepatology 73, 160–174 (2021).
Peng, Y. et al. The deubiquitylating enzyme USP15 regulates homologous recombination repair and cancer cell response to PARP inhibitors. Nat. Commun. 10, 1224 (2019).
Miller, S. & Muqit, M. M. K. Therapeutic approaches to enhance PINK1/Parkin mediated mitophagy for the treatment of Parkinson’s disease. Neurosci. Lett. 705, 7–13 (2019).
Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability — an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228 (2010).
Al-Hakim, A. et al. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair 9, 1229–1240 (2010).
Giovinazzi, S. et al. Usp7 protects genomic stability by regulating Bub3. Oncotarget 5, 3728–3742 (2014).
Valles, G. J., Bezsonova, I., Woodgate, R. & Ashton, N. W. USP7 is a master regulator of genome stability. Front. Cell Dev. Biol. 8, 717 (2020).
Rennie, M. L., Arkinson, C., Chaugule, V. K., Toth, R. & Walden, H. Structural basis of FANCD2 deubiquitination by USP1–UAF1. Nat. Struct. Mol. Biol. 28, 356–364 (2021).
Kim, J. M. et al. Inactivation of murine Usp1 results in genomic instability and a Fanconi anemia phenotype. Dev. Cell 16, 314–320 (2009).
Oestergaard, V. H. et al. Deubiquitination of FANCD2 is required for DNA crosslink repair. Mol. Cell 28, 798–809 (2007).
Das, D. S. et al. Blockade of deubiquitylating enzyme USP1 inhibits DNA repair and triggers apoptosis in multiple myeloma cells. Clin. Cancer Res. 23, 4280–4289 (2017).
Xu, X. et al. Inhibition of ubiquitin specific protease 1 sensitizes colorectal cancer cells to DNA-damaging chemotherapeutics. Front. Oncol. 9, 1406 (2019).
Huang, T. T. et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol. 8, 339–347 (2006). Together with Rennie et al. (2021), Kim et al. (2009), Oestergaard et al. (2007), Das et al. (2017) and Xu et al. (2019), this study represents a body of work revealing the targets and molecular mechanisms of USP1 and establishes its druggability that is now being tested in clinical trials.
Parsons, J. L. et al. USP47 is a deubiquitylating enzyme that regulates base excision repair by controlling steady-state levels of DNA polymerase β. Mol. Cell 41, 609–615 (2011).
Sobol, R. W. et al. Requirement of mammalian DNA polymerase-β in base-excision repair. Nature 379, 183–186 (1996).
Fan, Y. H. et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 4, e867 (2013).
Lei, H. et al. Targeting USP47 overcomes tyrosine kinase inhibitor resistance and eradicates leukemia stem/progenitor cells in chronic myelogenous leukemia. Nat. Commun. 12, 51 (2021).
McCann, J. J. et al. USP22 functions as an oncogenic driver in prostate cancer by regulating cell proliferation and DNA repair. Cancer Res. 80, 430–443 (2020).
Lambrus, B. G. et al. A USP28–53BP1–p53–p21 signaling axis arrests growth after centrosome loss or prolonged mitosis. J. Cell Biol. 214, 143–153 (2016).
Zhang, D., Zaugg, K., Mak, T. W. & Elledge, S. J. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 126, 529–542 (2006). This key paper reveals a role for USP28 in regulating DNA damage response through the CHK2–p53–PUMA pathway.
van den Berk, P. et al. USP15 deubiquitinase safeguards hematopoiesis and genome integrity in hematopoietic stem cells and leukemia cells. Cell Rep. 33, 108533 (2020).
Niederkorn, M. et al. The deubiquitinase USP15 modulates cellular redox and is a therapeutic target in acute myeloid leukemia. Leukemia 36, 438–451 (2022).
Teyra, J. et al. Structural and functional characterization of ubiquitin variant inhibitors of USP15. Structure 27, 590–605.e5 (2019).
Hermanns, T. et al. A family of unconventional deubiquitinases with modular chain specificity determinants. Nat. Commun. 9, 799 (2018).
Kwasna, D. et al. Discovery and characterization of ZUFSP/ZUP1, a distinct deubiquitinase class important for genome stability. Mol. Cell 70, 150–164.e6 (2018).
Haahr, P. et al. ZUFSP deubiquitylates K63-linked polyubiquitin chains to promote genome stability. Mol. Cell 70, 165–174 e166 (2018). Together with Kwasna et al. (2018), this study reveals a role for the previously uncharacterized DUB ZUP1 in maintaining genomic integrity through its ability to cleave Lys63-linked chains.
Leznicki, P. et al. Expansion of DUB functionality generated by alternative isoforms — USP35, a case study. J. Cell Sci. 131, e212753 (2018).
Ritorto, M. S. et al. Screening of DUB activity and specificity by MALDI-TOF mass spectrometry. Nat. Commun. 5, 4763 (2014). This important study developed mass-spectrometry-based screening to characterize DUB activities and revealed a lack of specificity of first-generation DUB inhibitor compounds.
Lange, S. M., Armstrong, L. A. & Kulathu, Y. Deubiquitinases: from mechanisms to their inhibition by small molecules. Mol. Cell 82, 15–29 (2022).
Gersch, M. et al. Distinct USP25 and USP28 oligomerization states regulate deubiquitinating activity. Mol. Cell 74, 436–451.e7 (2019).
Kategaya, L. et al. USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature 550, 534–538 (2017).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/study/NCT05240898 (2021).
Simoneau, A. et al. Ubiquitinated PCNA drives USP1 synthetic lethality in cancer. Mol. Cancer Ther. 22, 215–226 (2023).
Nijman, S. M. et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 17, 331–339 (2005).
Willson, J. DUBTACs for targeted protein stabilization. Nat. Rev. Drug. Discov. 21, 258 (2022).
Henning, N. J. et al. Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat. Chem. Biol. 18, 412–421 (2022). This paper establishes a proof of concept for redirecting DUBs to stabilize target proteins.
Liu, J. et al. TF-DUBTACs stabilize tumor suppressor transcription factors. J. Am. Chem. Soc. 144, 12934–12941 (2022).
Komander, D., Clague, M. J. & Urbe, S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563 (2009).
Faesen, A. C. et al. Mechanism of USP7/HAUSP activation by its C-terminal ubiquitin-like domain and allosteric regulation by GMP-synthetase. Mol. Cell 44, 147–159 (2011).
Zhao, Y. et al. OTUD4 is a phospho-activated K63 deubiquitinase that regulates MyD88-dependent signaling. Mol. Cell 69, 505–516.e5 (2018).
Zhao, Y. et al. Noncanonical regulation of alkylation damage resistance by the OTUD4 deubiquitinase. EMBO J. 34, 1687–1703 (2015).
de Poot, S. A. H., Tian, G. & Finley, D. Meddling with fate: the proteasomal deubiquitinating enzymes. J. Mol. Biol. 429, 3525–3545 (2017).
Worden, E. J., Padovani, C. & Martin, A. Structure of the Rpn11–Rpn8 dimer reveals mechanisms of substrate deubiquitination during proteasomal degradation. Nat. Struct. Mol. Biol. 21, 220–227 (2014).
Deol, K. K. et al. Proteasome-bound UCH37/UCHL5 debranches ubiquitin chains to promote degradation. Mol. Cell 80, 796–809.e9 (2020).
Zhang, S. et al. USP14-regulated allostery of the human proteasome by time-resolved cryo-EM. Nature 605, 567–574 (2022).
Yao, T. et al. Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Mol. Cell 31, 909–917 (2008).
Ernst, R., Mueller, B., Ploegh, H. L. & Schlieker, C. The otubain YOD1 is a deubiquitinating enzyme that associates with p97 to facilitate protein dislocation from the ER. Mol. Cell 36, 28–38 (2009).
Yuan, X. et al. Structure, dynamics and interactions of p47, a major adaptor of the AAA ATPase, p97. EMBO J. 23, 1463–1473 (2004).
Boeddrich, A. et al. An arginine/lysine-rich motif is crucial for VCP/p97-mediated modulation of ataxin-3 fibrillogenesis. EMBO J. 25, 1547–1558 (2006).
Atanassov, B. S. et al. ATXN7L3 and ENY2 coordinate activity of multiple H2B deubiquitinases important for cellular proliferation and tumor growth. Mol. Cell 62, 558–571 (2016).
Morgan, M. T. et al. Structural basis for histone H2B deubiquitination by the SAGA DUB module. Science 351, 725–728 (2016).
Atanassov, B. S. & Dent, S. Y. USP22 regulates cell proliferation by deubiquitinating the transcriptional regulator FBP1. EMBO Rep. 12, 924–930 (2011).
Roedig, J. et al. USP22 controls necroptosis by regulating receptor-interacting protein kinase 3 ubiquitination. EMBO Rep. 22, e50163 (2021).
Wiener, R. et al. E2 ubiquitin-conjugating enzymes regulate the deubiquitinating activity of OTUB1. Nat. Struct. Mol. Biol. 20, 1033–1039 (2013).
Wiener, R., Zhang, X., Wang, T. & Wolberger, C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature 483, 618–622 (2012).
Ji, L. et al. USP7 inhibits Wnt/β-catenin signaling through promoting stabilization of axin. Nat. Commun. 10, 4184 (2019).
Bushman, J. W. et al. Proteomics-based identification of DUB substrates using selective inhibitors. Cell Chem. Biol. 28, 78–87 e73 (2021). This important paper for the clinical translation of DUB inhibitors develops DUB inhibitor treatment coupled to mass spectrometry to profile DUB substrates.
Steger, M. et al. Time-resolved in vivo ubiquitinome profiling by DIA-MS reveals USP7 targets on a proteome-wide scale. Nat. Commun. 12, 5399 (2021).
Clancy, A. et al. The deubiquitylase USP9X controls ribosomal stalling. J. Cell Biol. 220, e202024211 (2021). This paper identifies an important role for USP9X in ribosomal stalling that may explain its importance in tumour settings.
Diefenbacher, M. E. et al. Usp28 counteracts Fbw7 in intestinal homeostasis and cancer. Cancer Res. 75, 1181–1186 (2015).
Diefenbacher, M. E. et al. The deubiquitinase USP28 controls intestinal homeostasis and promotes colorectal cancer. J. Clin. Invest. 124, 3407–3418 (2014).
Wang, X. et al. The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci. Rep. 6, 26979 (2016).
Rowinsky, E. K. et al. Phase 1 study of the protein deubiquitinase inhibitor VLX1570 in patients with relapsed and/or refractory multiple myeloma. Invest. New Drugs 38, 1448–1453 (2020).
Ward, J. A. et al. Re-evaluating the mechanism of action of α,β-unsaturated carbonyl DUB inhibitors b-AP15 and VLX1570: a paradigmatic example of unspecific protein cross-linking with michael acceptor motif-containing drugs. J. Med. Chem. 63, 3756–3762 (2020).
Acknowledgements
The authors thank S. Devine for help with compound structures. G.D. is supported by a fellowship from the Bodhi Education Foundation and grants from the National Health and Medical Research Council Australia (GNT2001406, GNT2004446). P.J.A.E.’s research was carried out during the tenure of a research project grant from Cancer Council Western Australia (CCWA 2022/1165) and WANMA (WANMA2021/7 Eichhorn). D.K. is supported by the National Health and Medical Research Council Australia (GNT1178122). The authors thank A. Wylie (KSQ Therapeutics, Cambridge, MA, USA) for sharing unpublished insights on KSQ-4279. This work was supported by operational infrastructure grants through the Australian Government Independent Research Institute Infrastructure Support Scheme (9000587) and the Victorian State Government Operational Infrastructure Support, Australia. The authors apologize to authors whose relevant work was not discussed due to space constraints.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
G.D. and D.K. are employees of the Walter and Eliza Hall Institute of Medical Research, which receives milestone payments for Venclexta (venetoclax). D.K. is founder and shareholder of Entact Bio. P.J.A.E. declares no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
FusionGDB: https://ccsm.uth.edu/FusionGDB/gene_search_result_0.cgi
Supplementary information
Glossary
- Alternative lengthening of telomeres
-
(ALT). A mechanism to limit degradation of telomeres that is independent of telomerase.
- Autophagosome
-
A double-membrane structure encapsulating autophagy cargo.
- Autophagy
-
The clearance of organelles or protein aggregates through lysosomal degradation.
- BH3 mimetics
-
Small molecules designed to mimic the activity of BH3-only proteins in binding pro-survival BCL-2 proteins.
- Condensate formation
-
The membrane-less compartmentalization of biomolecules driven by changes in solubility.
- Degron
-
A minimal protein signal for target protein degradation.
- E3 ubiquitin ligase enzymes
-
Enzymes that catalyse the transfer of ubiquitin to substrates.
- Fanconi anaemia DNA repair pathway
-
A multi-protein pathway to repair DNA inter-strand cross-links.
- Glutaminolysis
-
The conversion of glutamine into carbon sources for the TCA cycle.
- Hypomorphic variants
-
Amino acid substitutions that reduce, but do not block, protein function.
- Isopeptide bonds
-
A type of covalent bond between the carboxyl group of one amino acid and the amino group of another.
- Lipid peroxidation
-
The oxidative damage of lipids.
- Lysosome
-
Membrane-bound organelles containing enzymes to breakdown biomolecules, including protein and nucleic acids.
- Mitophagy
-
The autophagy of mitochondria.
- Non-homologous recombination
-
An error-prone DNA double strand break repair mechanism.
- Nucleotide excision repair
-
The bulk removal of mutagen-induced DNA lesions.
- Peroxisomes
-
An organelle that performs metabolic reactions and detoxifies oxygen species.
- Proteasome
-
A multisubunit machine for the selective degradation of proteins.
- Scissile bond
-
A covalent bond that can be broken by an enzyme.
- Spindle checkpoint
-
A complex that ensures proper segregation of duplicate chromosomes during mitosis or meiosis.
- Synthetic lethality
-
Mutations or alterations in multiple genes that are lethal when in combination, but not alone.
- Telomerase
-
An enzyme that adds repetitive sequence to telomeres to maintain telomere length at chromosome ends.
- Translesion synthesis
-
DNA synthesis across a lesion to avoid DNA replication failure.
- Unfoldases
-
Enzymes that promote protein unfolding.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dewson, G., Eichhorn, P.J.A. & Komander, D. Deubiquitinases in cancer. Nat Rev Cancer 23, 842–862 (2023). https://doi.org/10.1038/s41568-023-00633-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00633-y
This article is cited by
-
TRIM25 promotes glioblastoma cell growth and invasion via regulation of the PRMT1/c-MYC pathway by targeting the splicing factor NONO
Journal of Experimental & Clinical Cancer Research (2024)