Abstract
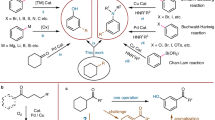
This protocol describes an approach to installing hydroxyls into arenes through the direct replacement of C–H bonds with C–O bonds. This direct oxidation avoids the need to prefunctionalize the substrate, use precious metals, introduce directing groups, or use strong Brønsted or Lewis acids. Phthaloyl peroxide, the sole reagent used for this transformation, can be prepared readily from the commodity chemicals phthaloyl chloride and sodium percarbonate. Phthaloyl peroxide oxidizes a diverse range of arenes, and the reactions that involve its use are characterized by high functional group compatibility, which enables the hydroxylation of simple arenes, advanced synthetic intermediates, natural products and other drug-like molecules forming the corresponding phenolic compounds. Notably, the reaction is operationally straightforward and has no special requirements for the exclusion of oxygen and water. The synthesis of phthaloyl peroxide takes 4 h and the subsequent hydroxylation of mesitylene takes 21 h.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wender, P.A., Verma, V.A., Paxton, T.J. & Pillow, T.H. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 41, 40–49 (2008).
Derbyshire, D.H. & Waters, W.A. An oxidation involving the hydroxyl cation, (OH)+. Nature 165, 401 (1950).
Kovacic, P. & Kurz, M.E. Friedel-Crafts oxygenation of anisole and alkylbenzenes with diisopropyl peroxydicarbonate. J. Am. Chem. Soc. 87, 4811–4818 (1965).
Vesely, J.A. & Schmerli, L. Hydrogen fluoride catalyzed hydroxylation of aromatic compounds. J. Org. Chem. 35, 4028–4033 (1970).
Olah, G.A. & Ohnishi, R. Oxyfunctionalization of hydrocarbons VIII. Electrophilic hydroxylation of benzene, alkylbenzenes, and halobenzenes with hydrogen-peroxide in superacids. J. Org. Chem. 43, 865–867 (1978).
Yuan, C. et al. Metal-free oxidation of aromatic carbon-hydrogen bonds through a reverse-rebound mechanism. Nature 499, 192–196 (2013).
Greene, F.D. Cyclic diacyl peroxides I. Monomeric phthaloyl peroxide. J. Am. Chem. Soc. 78, 2246–2250 (1956).
Russell, K.E. The preparation of phthalyl peroxide and its decomposition in solution. J. Am. Chem. Soc. 77, 4814–4815 (1955).
Mckillop, A. & Sanderson, W.R. Sodium perborate and sodium percarbonate: cheap, safe and versatile oxidizing agents for organic synthesis. Tetrahedron 51, 6145–6166 (1995).
Rocha Gonsalves, A.M.d'A., Johnstone, R.A.W., Pereira, M.M. & Shaw, J. Dissociation of hydrogen peroxide adducts in solution: the use of such adducts for epoxidation of alkenes. J. Chem. Res. (M) 2101–2118 (1991).
Yuan, C.X., Axelrod, A., Varela, M., Danysh, L. & Siegel, D. Synthesis and reaction of phthaloyl peroxide derivatives, potential organocatalysts for the stereospecific dihydroxylation of alkenes. Tet. Lett. 52, 2540–2542 (2011).
Begue, J.P., Bonnet-Delpon, D. & Crousse, B. Fluorinated alcohols: a new medium for selective and clean reaction. Synlett 18–29 (2004).
Shuklov, I.A., Dubrovina, N.V. & Boerner, A. Fluorinated alcohols as solvents, cosolvents and additives in homogeneous catalysis. Synthesis 2925–2943 (2007).
Andres, G.O., Granados, A.M. & de Rossi, R.H. Kinetic study of the hydrolysis of phthalic anhydride and aryl hydrogen phthalates. J. Org. Chem. 66, 7653–7657 (2001).
Mahoney, K.M., Goswami, P.P. & Winter, A.H. Self-immolative aryl phthalate esters. J. Org. Chem. 78, 702–705 (2013).
Still, W.C., Kahn, M. & Mitra, A. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 43, 2923–2925 (1978).
Acknowledgements
Financial support from the Welch Foundation (F-1694) is gratefully acknowledged. The authors thank C. Bielawski and A. Teator for assistance with Karl Fischer titrations.
Author information
Authors and Affiliations
Contributions
C.Y., A.E. and A.C. designed the experiments and carried out the experiments; C.Y., A.E., A.C. and D.S. analyzed the data and wrote the paper; and D.S. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yuan, C., Eliasen, A., Camelio, A. et al. Preparation of phenols by phthaloyl peroxide–mediated oxidation of arenes. Nat Protoc 9, 2624–2629 (2014). https://doi.org/10.1038/nprot.2014.175
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.175
This article is cited by
-
Electrochemical behavior of phthaloyl peroxide in aqueous media
Russian Chemical Bulletin (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.