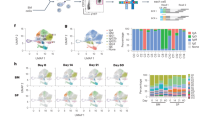
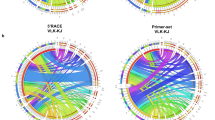
Abstract
The ability to rapidly generate large panels of antigen-specific human antibodies in a rodent would enable the efficient discovery of novel therapeutically useful antibodies. We have developed a system wherein human antigen-specific antibody–secreting plasmablasts can be enriched in vivo, in a severe combined immunodeficient (SCID)/beige mouse host. The antigen-specific plasmablasts can then be sorted by flow cytometry, enabling single-cell cloning and expression of fully human immunoglobulin-G. By using this technique, we have generated four broadly reactive anti–influenza A antibodies. Therefore, the method described here is useful for the identification of rare functional antibodies. This protocol takes ∼1 month to complete, from the time of human vaccination to the cloning of heavy- and light-chain genes. For additional small-scale transient expression, purification and binding analysis, the protocol would take an additional month.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nakamura, G. et al. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe 14, 93–103 (2013).
Brams, P., Nguyen, M., Chamat, S., Royston, I. & Morrow, P.R. Antigen-specific IgG responses from naive human splenocytes: in vitro priming followed by antigen boost in the SCID mouse. J. Immunol. 160, 2051–2058 (1998).
Depraetere, S., Verhoye, L., Leclercq, G. & Leroux-Roels, G. Human B cell growth and differentiation in the spleen of immunodeficient mice. J. Immunol. 166, 2929–2936 (2001).
Klein, B. et al. Survival and proliferation factors of normal and malignant plasma cells. Int. J. Hematol. 78, 106–113 (2003).
Odendahl, M. et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105, 1614–1621 (2005).
Chen, G.J., Qiu, N., Karrer, C., Caspers, P. & Page, M.G. Restriction site-free insertion of PCR products directionally into vectors. Biotechniques 28, 498–500, 504–505 (2000).
Geiser, M., Cebe, R., Drewello, D. & Schmitz, R. Integration of PCR fragments at any specific site within cloning vectors without the use of restriction enzymes and DNA ligase. Biotechniques 31, 88–90, 92 (2001).
van den Ent, F. & Löwe, J. RF cloning: a restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 67, 67–74 (2006).
Li, M.Z. & Elledge, S.J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 4, 251–256 (2007).
Bryksi, A. & Matsumura, I. Overlap extension PCR cloning: a simple and reliable way to create recombinant plasmids. Biotechniques 48, 463–465 (2010).
Unger, T., Jacobovitch, Y., Dantes, A., Bernheim, R. & Peleg, Y. Applications of the restriction free (RF) cloning procedure for molecular manipulations and protein expression. J. Struct. Biol. 172, 34–44 (2010).
Bond, S. & Naus, C. RF-cloning.org: an online tool for the design of restriction-free cloning projects. Nucleic Acids Res. 40, 209–213 (2012).
Acknowledgements
We are grateful for the flow cytometry expertise of J. Cupp, L. Gilmour, J. Borneo, C.K. Poon and T. Ho. We also thank E. Brown and F. Martin for insightful discussion. We thank Y. Yang, A. Wong, K. Billeci, S. Heldens, P. Hass and K. Schroeder for their technical expertise.
Author information
Authors and Affiliations
Contributions
Z.L., N.Y.C., N.C., W.P.L., M.B. and G.N. performed the experiments; L.R.S., M.B., N.C., G.N. and N.Y.C. developed the methods; and Z.L., G.N., D.S. and L.R.S. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
At the time of this work, Z.L., N.Y.C., N.C., W.P.L., M.B., G.N. and L.R.S. were employees of Genentech, a member of the Roche Group, and may have an equity interest in Roche.
Rights and permissions
About this article
Cite this article
Lin, Z., Chiang, N., Chai, N. et al. In vivo antigen-driven plasmablast enrichment in combination with antigen-specific cell sorting to facilitate the isolation of rare monoclonal antibodies from human B cells. Nat Protoc 9, 1563–1577 (2014). https://doi.org/10.1038/nprot.2014.104
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.104
This article is cited by
-
Human monoclonal antibodies isolated from a primary pneumococcal conjugate Vaccinee demonstrates the expansion of an antigen-driven Hypermutated memory B cell response
BMC Infectious Diseases (2018)
-
A broadly protective therapeutic antibody against influenza B virus with two mechanisms of action
Nature Communications (2017)
-
Reconstituted B cell receptor signaling reveals carbohydrate-dependent mode of activation
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.