Abstract
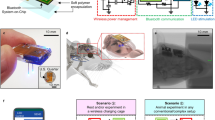
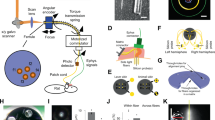
The rise of optogenetics provides unique opportunities to advance materials and biomedical engineering, as well as fundamental understanding in neuroscience. This protocol describes the fabrication of optoelectronic devices for studying intact neural systems. Unlike optogenetic approaches that rely on rigid fiber optics tethered to external light sources, these novel devices carry wirelessly powered microscale, inorganic light-emitting diodes (μ-ILEDs) and multimodal sensors inside the brain. We describe the technical procedures for construction of these devices, their corresponding radiofrequency power scavengers and their implementation in vivo for experimental application. In total, the timeline of the procedure, including device fabrication, implantation and preparation to begin in vivo experimentation, can be completed in ∼3–8 weeks. Implementation of these devices allows for chronic (tested for up to 6 months) wireless optogenetic manipulation of neural circuitry in animals navigating complex natural or home-cage environments, interacting socially, and experiencing other freely moving behaviors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Yizhar, O., Fenno, L.E., Davidson, T.J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Carter, M.E et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 13, 1526–1533 (2010).
Stuber, G.D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011).
Jennings, J.H. et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013).
Kim, S.-Y. et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Tye, K.M. et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).
Kim, T.-i. et al. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340, 211–216 (2013).
Kim, T.-i. et al. High-efficiency, microscale GaN light-emitting diodes and their thermal properties on unusual substrates. Small 8, 1643–1649 (2012).
Kim, D.-H. et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 9, 511–517 (2010).
Kim, T.-i., Kim, R.-H. & Rogers, J.A. Microscale inorganic light-emitting diodes on flexible and stretchable substrates. IEEE Photonics J. 4, 607–612 (2012).
Al-Hasani, R., McCall, J.G., Foshage, A.M. & Bruchas, M.R. Locus coeruleus kappa opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology 38, 2484–2497 (2013).
Li, Y. et al. Thermal analysis of injectable, cellular-scale optoelectronics with pulsed power. Proc. R. Soc. Math. Phys. Eng. Sci. 469, 20130142 (2013).
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012).
Tao, H. et al. Silk-based conformal, adhesive, edible food sensors. Adv. Mater. 24, 1067–1072 (2012).
Cao, H., Gu, L., Mohanty, S.K. & Chiao, J.-C. An integrated μLED optrode for optogenetic stimulation and electrical recording. IEEE Trans. Biomed. Eng. 60, 225–229 (2013).
Wentz, C.T. et al. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J. Neural Eng. 8, 046021 (2011).
Iwai, Y., Honda, S., Ozeki, H., Hashimoto, M. & Hirase, H. A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci. Res. 70, 124–127 (2011).
Zhao, Y., Larimer, P., Pressler, R.T., Strowbridge, B.W. & Burda, C. Wireless activation of neurons in brain slices using nanostructured semiconductor photoelectrodes. Angew. Chem. Int. Ed. 48, 2407–2410 (2009).
Sparta, D.R. et al. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat. Protoc. 7, 12–23 (2011).
Zorzos, A.N., Boyden, E.S. & Fonstad, C.G. Multiwaveguide implantable probe for light delivery to sets of distributed brain targets. Opt. Lett. 35, 4133–4135 (2010).
Zorzos, A.N., Scholvin, J., Boyden, E.S. & Fonstad, C.G. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt. Lett. 37, 4841–4843 (2012).
Stark, E., Koos, T. & Buzsaki, G. Diode probes for spatiotemporal optical control of multiple neurons in freely moving animals. J. Neurophysiol. 108, 349–363 (2012).
Airan, R.D., Thompson, K.R., Fenno, L.E., Bernstein, H. & Deisseroth, K. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009).
Park, S.-I. et al. Printed assemblies of inorganic light-emitting diodes for deformable and semitransparent displays. Science 325, 977–981 (2009).
Diester, I. et al. An optogenetic toolbox designed for primates. Nat. Neurosci. 14, 387–397 (2011).
Gerits, A. & Vanduffel, W. Optogenetics in primates: a shining future? Trends Genet. (2013); 29, 403–411.
Cavanaugh, J. et al. Optogenetic inactivation modifies monkey visuomotor behavior. Neuron 76, 901–907 (2012).
Ruiz, O. et al. Optogenetics through windows on the brain in the nonhuman primate. J. Neurophysiol. (2013); 110, 1455–1467.
Witten, I.B. et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72, 721–733 (2011).
Han, X. et al. Millisecond-timescale optical control of neural dynamics in the nonhuman primate brain. Neuron 62, 191–198 (2009).
Kravitz, A.V., Owen, S.F. & Kreitzer, A.C. Optogenetic identification of striatal projection neuron subtypes during in vivo recordings. Brain Res. 1511, 21–32 (2013).
Szuts, T.A. et al. A wireless multi-channel neural amplifier for freely moving animals. Nat. Neurosci. 14, 263–269 (2011).
Harrison, R.R. et al. A wireless neural/EMG telemetry system for freely moving insects. Circuits Syst. ISCAS Proc. 2010 IEEE Int. Symp. 2940–2943 (2010).
Adamantidis, A.R. et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. Off. J. Soc. Neurosci. 31, 10829–10835 (2011).
Sparta, D.R., Jennings, J.H., Ung, R.L. & Stuber, G.D. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav. Brain Res. 15, 19–25 (2013).
Tye, K.M. & Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 13, 251–266 (2012).
Cold Spring Harbor Laboratory Press. Artificial cerebrospinal fluid (ACSF). Cold Spring Harb. Protoc. 2011 pdb.rec065730 (2011).
Du Hoffmann, J., Kim, J.J. & Nicola, S.M. An inexpensive drivable cannulated microelectrode array for simultaneous unit recording and drug infusion in the same brain nucleus of behaving rats. J. Neurophysiol. 106, 1054–1064 (2011).
Wong, W.S. et al. InxGa1–xN light emitting diodes on Si substrates fabricated by Pd–In metal bonding and laser lift-off. Appl. Phys. Lett. 77, 2822–2824 (2000).
Cardin, J.A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Gradinaru, V. et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell 141, 154–165 (2010).
Osakada, F. et al. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71, 617–631 (2011).
Osakada, F. & Callaway, E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 8, 1583–1601 (2013).
Clark, J.J. et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 7, 126–129 (2009).
Kim, R.-H. et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nat. Mater. 9, 929–937 (2010).
Song, Y.M. et al. Digital cameras with designs inspired by the arthropod eye. Nature 497, 95–99 (2013).
Acknowledgements
This work is supported by the US National Institutes of Health Common Fund; the National Institute on Drug Abuse (NIDA) grant no. R01DA037152 (M.R.B.); NIDA grant no. R00DA025182 (M.R.B.); National Institute of Neurological Disorders and Stroke (NINDS) grant no. R01NS081707 (M.R.B., J.A.R.); National Institute of Mental Health (NIMH) grant no. F31MH101956 (J.G.M.); the McDonnell Center for Systems Neuroscience (M.R.B.); a National Security Science and Engineering Faculty Fellowship of Energy (J.A.R.); the US Department of Energy, Division of Materials Sciences under award no. DE-FG02-07ER46471 (J.A.R.); the Materials Research Laboratory and Center for Microanalysis of Materials (DE-FG02-07ER46453) (J.A.R.) and the Washington University in St. Louis Division of Biological and Biomedical Sciences (J.G.M.); and the Institute for Basic Science (IBS) (T-i.K.) and National Research foundation of Korea Grant funded by the Ministry of Science, ICT and Future Planning (2009-0083540) in Korea (T-i.K.). We thank H. Tao (Tufts University) and S. Hwang (University of Illinois at Urbana-Champaign) for their help in the preparation of silk solution and for valuable discussions. We thank A.M. Foshage, E.R. Siuda and other members of the Bruchas laboratory, the laboratory of R.W. Gereau, IV (Washington University in St. Louis) and the laboratory of G.D. Stuber (University of North Carolina) for helpful discussion and technical advice. We also thank K. Deisseroth (Stanford University) for the channelrhodopsin-2 (H134) constructs, the Stuber laboratory for the TH-IRES-Cre mice, the Washington University in St. Louis Bakewell Neuroimaging Laboratory Core, the Washington University in St. Louis Machine Shop and the Washington University in St. Louis` Hope Center Viral Vector Core.
Author information
Authors and Affiliations
Contributions
J.G.M., T-i.K., G.S., X.H., Y.H.J. and R.A.-H. performed the experiments. J.G.M., T-i.K., G.S., X.H., R.A.-H., F.G.O., M.R.B. and J.A.R. developed the protocol. J.G.M., T-i.K., G.S., X.H., M.R.B. and J.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Machining of the cannula holder adapter.
This adapter is specifically designed for use with the KOPF Model 1966 Cannula Holder. The adapter is fashioned from aluminum with an 8 mm stalk (3 mm in diameter) that can be held by the Model 1966. The main body of the adapter is 14 mm in length with a 7 mm diameter. There are two orthogonal bore holes through the body. The first is a 5 mm hole from which the center slit is created through to the tip of the adapter. The second is a 2 mm screw-hole so that a screw can be tightened to reduce the size of the center slit to hold the μ-needle. It is important that the center point of the adapter be in-line with the center point of the cannula holder itself to ensure accurate device injection. Note that this adapter is merely a suggestion, but we acknowledge there can be many other solutions to the problem of accurate injection of the devices. Most stereotaxic instrument manufacturers offer custom-built holders and it is likely that many standard electrode holders can be modified to suit the needs of the individual laboratory (e.g. KOPF Model 1768).
Supplementary information
Supplementary Figure 1
(PDF 132 kb)
Rights and permissions
About this article
Cite this article
McCall, J., Kim, Ti., Shin, G. et al. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat Protoc 8, 2413–2428 (2013). https://doi.org/10.1038/nprot.2013.158
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.158
This article is cited by
-
Body-conformable light-emitting materials and devices
Nature Photonics (2024)
-
Customizable, wireless and implantable neural probe design and fabrication via 3D printing
Nature Protocols (2023)
-
Multifunctional microelectronic fibers enable wireless modulation of gut and brain neural circuits
Nature Biotechnology (2023)
-
Self-assembled ultraflexible probes for long-term neural recordings and neuromodulation
Nature Protocols (2023)
-
Flexible high-resolution micro-LED display device with integrations of transparent, conductive, and highly elastic hydrogel
Nano Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.