Abstract
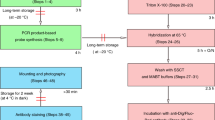
Histological techniques are critical for observing tissue and cellular morphology. In this paper, we outline our protocol for embedding, serial sectioning, staining and visualizing zebrafish embryos embedded in JB-4 plastic resin—a glycol methacrylate-based medium that results in excellent preservation of tissue morphology. In addition, we describe our procedures for staining plastic sections with toluidine blue or hematoxylin and eosin, and show how to couple these stains with whole-mount RNA in situ hybridization. We also describe how to maintain and visualize immunofluorescence and EGFP signals in JB-4 resin. The protocol we outline—from embryo preparation, embedding, sectioning and staining to visualization—can be accomplished in 3 d. Overall, we reinforce that plastic embedding can provide higher resolution of cellular details and is a valuable tool for cellular and morphological studies in zebrafish.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kimmel, C.B., Ballard, W.W., Kimmel, S.R., Ullmann, B. & Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310 (1995).
Du, S.J., Frenkel, V., Kindschi, G. & Zohar, Y. Visualizing normal and defective bone development in zebrafish embryos using the fluorescent chromophore calcein. Dev. Biol. 238, 239–246 (2001).
Okabe, N., Xu, B. & Burdine, R.D. Fluid dynamics in zebrafish Kupffer's vesicle. Dev. Dyn. 237, 3602–3612 (2008).
Schottenfeld, J., Sullivan-Brown, J. & Burdine, R.D. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development 134, 1605–1615 (2007).
Serluca, F.C. et al. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning. Development 136, 1621–1631 (2009).
Sullivan-Brown, J. et al. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev. Biol. 314, 261–275 (2008).
Weber, S. et al. SIX2 and BMP4 mutations associate with anomalous kidney development. J. Am. Soc. Nephrol. 19, 891–903 (2008).
Baker, K., Holtzman, N.G. & Burdine, R.D. Direct and indirect roles for Nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proc. Natl. Acad. Sci. USA 105, 13924–13929 (2008).
Macdonald, R. Zebrafish immunohistochemistry. Methods Mol. Biol. 127, 77–88 (1999).
Sabaliauskas, N.A. et al. High-throughput zebrafish histology. Methods 39, 246–254 (2006).
Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). 4th edn. (University of Oregon Press, 2000).
Bancroft, J.D. & Gamble, M. Theory and Practice of Histological Techniques, 6th edn. (Churchill Livingstone, 2008).
Thisse, C. & Thisse, B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69 (2008).
Macintosh, F.C. A colorimetric method for the standardization of heparin preparations. Biochem. J. 35, 776–782 (1941).
Wheater, P.R., Burkitt, H.G. & Daniels, V.G. Functional Histology: A Text and Colour Atlas, 2nd edn. (Churchill Livingstone, 1987).
Scott, A. & Stemple, D.L. Zebrafish notochordal basement membrane: signaling and structure. Curr. Top. Dev. Biol. 65, 229–253 (2005).
Yelon, D., Horne, S.A. & Stainier, D.Y. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 214, 23–37 (1999).
diIorio, P.J., Moss, J.B., Sbrogna, J.L., Karlstrom, R.O. & Moss, L.G. Sonic hedgehog is required early in pancreatic islet development. Dev. Biol. 244, 75–84 (2002).
Okada, A., Lansford, R., Weimann, J.M., Fraser, S.E. & McConnell, S.K. Imaging cells in the developing nervous system with retrovirus expressing modified green fluorescent protein. Exp. Neurol. 156, 394–406 (1999).
Huang, C.J., Tu, C.T., Hsiao, C.D., Hsieh, F.J. & Tsai, H.J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 228, 30–40 (2003).
Acknowledgements
We thank K. Baker, K. Jaffe, N. Okabe, J. Rosen and J. Schottenfeld for providing samples and help with the protocol; H.J. Tsai for providing Tg(myl7:EGFP)twu34 strain; M. Pack for advice on immunofluorescence protocols; V. Grover for information on storage conditions for staining reagents; and members of the Burdine lab for critical reading of the manuscript. R.D.B. is the 44th Scholar of the Edward Mallinckrodt, Jr. Foundation, and funds from this award were used in support of this work. Funds from awards to R.D.B. from the New Jersey Commission on Cancer Research (04-2405-CCR-E0), the Polycystic Kidney Disease Foundation (no. 117b2r), and from the National Institute of Child Health and Human Development (R01-HD048584) were used in support of this work. J.S.-B. was supported by predoctoral award 05-2411-CCR-E0 from the New Jersey Commission on Cancer Research.
Author information
Authors and Affiliations
Contributions
J.S.-B. and M.E.B. developed the protocol. R.D.B. supervised the work. J.S.-B., M.E.B. and R.D.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sullivan-Brown, J., Bisher, M. & Burdine, R. Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nat Protoc 6, 46–55 (2011). https://doi.org/10.1038/nprot.2010.165
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.165
This article is cited by
-
Generation of iPSC-derived human forebrain organoids assembling bilateral eye primordia
Nature Protocols (2023)
-
Enhancement of zebrafish sperm production via a large body-sized surrogate with germ cell transplantation
Communications Biology (2023)
-
Semaphorin3f as a cardiomyocyte derived regulator of heart chamber development
Cell Communication and Signaling (2022)
-
Comparative demography of four large-bodied surgeonfish
Environmental Biology of Fishes (2022)
-
Collecting near mature and immature orchid seeds for ex situ conservation: ‘in vitro collecting’ as a case study
Botanical Studies (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.