Abstract
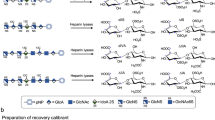
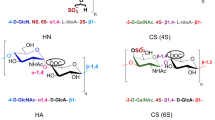
One of the first steps in characterizing heparan sulfate (HS) and its close relative heparin is to conduct disaccharide composition analysis. This provides an overall picture of the structure of the polysaccharide in terms of its constituent disaccharides. This is of importance, for example, in the initial characterization of spatially and temporally regulated structures. Two protocols for conducting disaccharide analysis are presented here, both exploiting exhaustive digestion of the polysaccharide, yielding constituent disaccharides, by bacterial heparin lyases. The first method, suitable for microgram quantities of material, relies on the separation of the disaccharides by high-performance liquid chromatography (HPLC) coupled to ultraviolet absorbance detection and can be performed in 2 d. The second exploits reducing end–labeling with the fluorophore BODIPY hydrazide, separation by HPLC, and subsequent fluorescence detection and quantitation. The latter is a high-sensitivity method that requires nanograms of starting material and has a detection limit in the low fmol range, and is thus the most sensitive method for disaccharide compositional analysis of HS yet reported. Fluorescence detection can be routinely carried out in 3 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Raman, R., Raguram, S., Venkataraman, G., Paulson, J.C. & Sasisekharan, R. Glycomics: an integrated systems approach to structure-function relationships of glycans. Nat. Methods 2, 817–824 (2005).
Turnbull, J.E. & Field, R.A. Emerging glycomics technologies. Nat. Chem. Biol. 3, 74–77 (2007).
Bishop, J.R., Schuksz, M. & Esko, J.D. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 (2007).
Turnbull, J., Powell, A. & Guimond, S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11, 75–82 (2001).
Rabenstein, D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 19, 312–331 (2002).
Skidmore, M.A. & Turnbull, J.E. in Chemistry and Biology of Heparin and Heparan Sulphate (eds. Garg, H.G., Linhardt, R.J. & Hales, C.A.) 179–202 (Elsevier, New York, 2005).
Turnbull, J.E. & Gallagher, J.T. Distribution of iduronate 2-sulphate residues in heparan sulphate. Evidence for an ordered polymeric structure. Biochem. J. 273 (Part 3), 553–559 (1991).
Scholefield, Z. et al. Heparan sulfate regulates amyloid precursor protein processing by BACE1, the Alzheimer's beta-secretase. J. Cell Biol. 163, 97–107 (2003).
Skidmore, M.A. et al. Disruption of rosetting in Plasmodium falciparum malaria with chemically modified heparin and low molecular weight derivatives possessing reduced anticoagulant and other serine protease inhibition activities. J. Med. Chem. 51, 1453–1458 (2008).
Vogt, A.M. et al. Heparan sulfate on endothelial cells mediates the binding of Plasmodium falciparum-infected erythrocytes via the DBL1alpha domain of PfEMP1. Blood 101, 2405–2411 (2003).
Lever, R. & Page, C.P. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 1, 140–148 (2002).
Guimond, S. & Turnbull, J.E. Proteoglycans make the grade-ient. Mol. Cell 16, 159–160 (2004).
Powell, A.K., Yates, E.A., Fernig, D.G. & Turnbull, J.E. Interactions of heparin/heparan sulfate with proteins: appraisal of structural factors and experimental approaches. Glycobiology 14, 17R–30R (2004).
Guimond, S.E. & Turnbull, J.E. Fibroblast growth factor receptor signalling is dictated by specific heparan sulphate saccharides. Curr. Biol. 9, 1343–1346 (1999).
Ford-Perriss, M. et al. Variant heparan sulfates synthesized in developing mouse brain differentially regulate FGF signaling. Glycobiology 12, 721–727 (2002).
Powell, A.K., Ahmed, Y.A., Yates, E.A. & Turnbull, J.E. Generating heparan sulfate saccharide libraries for glycomics applications. Nat. Protoc. 5, 821–833 (2010).
Volpi, N. & Linhardt, R.J. High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides. Nat. Protoc. 5, 993–1004 (2010).
Guimond, S.E. et al. Rapid purification and high sensitivity analysis of heparan sulfate from cells and tissues: toward glycomics profiling. J. Biol. Chem. 284, 25714–25722 (2009).
Linhardt, R.J., Turnbull, J.E., Wang, H.M., Loganathan, D. & Gallagher, J.T. Examination of the substrate-specificity of heparin and heparan-sulfate lyases. Biochemistry 29, 2611–2617 (1990).
Turnbull, J.E., Hopwood, J.J. & Gallagher, J.T. A strategy for rapid sequencing of heparan sulfate and heparin saccharides. Proc. Natl. Acad. Sci. USA 96, 2698–2703 (1999).
Deakin, J.A. & Lyon, M. A simplified and sensitive fluorescent method for disaccharide analysis of both heparan sulfate and chondroitin/dermatan sulfates from biological samples. Glycobiology 18, 483–491 (2008).
Drummond, K.J., Yates, E.A. & Turnbull, J.E. Electrophoretic sequencing of heparin/heparan sulfate oligosaccharides using a highly sensitive fluorescent end label. Proteomics 1, 304–310 (2001).
Haughland, R.P., Steinberg, T.H., Patton, W.F., Diwu, Z. Reagents for labeling biomolecules having aldehyde or ketone moieties. US Patent 6967251 (2005).
Huang, Y. et al. Simultaneous determination of dermatan sulfate and oversulfated dermatan sulfate in plasma by high-performance liquid chromatography with postcolumn fluorescence derivatization. Anal. Biochem. 240, 227–234 (1996).
Skidmore, M.A. et al. High sensitivity separation and detection of heparan sulfate disaccharides. J. Chromatogr. A 1135, 52–56 (2006).
Toyoda, H., Yamamoto, H., Ogino, N., Toida, T. & Imanari, T. Rapid and sensitive analysis of disaccharide composition in heparin and heparan sulfate by reversed-phase ion-pair chromatography on a 2 μm porous silica gel column. J. Chromatogr. A 830, 197–201 (1999).
Kinoshita, A. & Sugahara, K. Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high-performance liquid chromatography: application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal. Biochem. 269, 367–378 (1999).
Ambrosius, M., Kleesiek, K. & Gotting, C. Quantitative determination of the glycosaminoglycan Delta-disaccharide composition of serum, platelets and granulocytes by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1201, 54–60 (2008).
Skidmore, M.A., Atrih, A. & Yates, E.A. Labelling heparan sulphate saccharides with chromophore, fluorescence and mass tags for HPLC and MS separations. Methods Mol. Biol. 534, 157–169 (2009).
Acknowledgements
This study was funded by grants from the Biotechnology and Biological Sciences Research Council (J.E.T., M.A.S., S.E.G. and E.A.Y.), the Wellcome Trust (J.E.T., M.A.S., S.E.G. and E.A.Y.), the Human Frontier Science Program (J.E.T.), the Royal Society (E.A.Y. and M.A.S.) and the Medical Research Council (Senior Research Fellowship to J.E.T.).
Author information
Authors and Affiliations
Contributions
M.A.S. developed the fluorescence labeling methodology and associated SAX HPLC separation, performed experiments and wrote the article. J.E.T. developed the SAX HPLC methodology for UV detection. S.E.G. and A.F.D. performed labeling and SAX experiments. J.E.T. and E.A.Y. obtained funding, edited the article and provided overall supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Skidmore, M., Guimond, S., Dumax-Vorzet, A. et al. Disaccharide compositional analysis of heparan sulfate and heparin polysaccharides using UV or high-sensitivity fluorescence (BODIPY) detection. Nat Protoc 5, 1983–1992 (2010). https://doi.org/10.1038/nprot.2010.145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.145
This article is cited by
-
Chemical O-sulfation of N-sulfoheparosan: a route to rare N-sulfo-3-O-sulfoglucosamine and 2-O-sulfoglucuronic acid
Glycoconjugate Journal (2020)
-
Surveying silicon nitride nanopores for glycomics and heparin quality assurance
Nature Communications (2018)
-
A mutant-cell library for systematic analysis of heparan sulfate structure–function relationships
Nature Methods (2018)
-
Glycosaminoglycanomics: where we are
Glycoconjugate Journal (2017)
-
Site-specific identification of heparan and chondroitin sulfate glycosaminoglycans in hybrid proteoglycans
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.