Abstract
Heroin and cocaine have very different unconditioned receptor-mediated actions; however, in the brain circuitry of drug-reward and motivation, the two drugs establish common conditioned consequences. A single experience with either drug can change the sensitivity of ventral tegmental area (VTA) dopamine neurons to glutamatergic input. In the case of cocaine, repeated intravenous self-administration establishes de novo VTA glutamate release and dopaminergic activation in response to conditioned stimuli and mild footshock stress. Here we determined whether repeated self-administration of heroin would establish similar glutamate release and dopaminergic activation. Although self-administration of heroin itself did not cause VTA glutamate release, conditioned glutamate release was seen when rats expecting rewarding heroin were given nonrewarding saline in its place. Mild footshock stress also caused glutamate release in heroin-trained animals. In each case, the VTA glutamate release was accompanied by elevations in VTA dopamine levels, indicative of dopaminergic activation. In each case, infusion of the ionotropic glutamate antagonist kynurenic acid blocked the VTA dopamine release associated with VTA glutamate elevation. Although glutamate levels in the extinction and reinstatement tests were similar to those reported in cocaine studies, the effects of heroin self-administration itself were quite different from what has been seen during cocaine self-administration.
Similar content being viewed by others
INTRODUCTION
The habit-forming (reinforcing) properties of most (Wise, 2008; Wise and Bozarth, 1981; Wise and Rompré, 1989), but not all (Carlezon and Wise, 1996), drugs of abuse have been linked to the ability of these drugs to activate the mesocorticolimbic dopamine system. Activation of this system also contributes to the instigation of drug taking after periods of abstinence (Anderson et al, 2003; Schmidt and Pierce, 2006; Wise et al, 1990). Major excitatory input to the system comes from multiple sources of glutamatergic afferents to the ventral tegmental area (VTA) (Geisler and Wise, 2008; Geisler and Zahm, 2005). Glutamate antagonists perfused into the VTA block the cocaine seeking caused by mild footshock stress (Wang et al, 2005), by cocaine-predictive environmental stimuli (You et al, 2007), and by priming injections of cocaine itself (Wise et al, 2008).
The role of VTA glutamate in heroin seeking has not been investigated in any detail beyond the finding that ventral tegmental infusion of glutamate antagonists attenuate opiate reward (Shabat-Simon et al, 2008; Xi and Stein, 2002). Opiates have rewarding actions in the VTA (Bozarth and Wise, 1981; Devine and Wise, 1994; Phillips and LePiane, 1980; Zangen et al, 2002), and the rewarding actions of μ- and δ-opioids are in proportion to their ability to activate the dopamine system (Devine et al, 1993). However, the ability of opiates to activate the dopamine system is thought to be mediated by inhibition of GABAergic input (Johnson and North, 1992; Matsui and Williams, 2011) rather than by excitation of glutamate input to the dopamine system. Indeed, opiates tend to inhibit glutamate input onto VTA dopamine neurons (Margolis et al, 2005). Nonetheless, in the case of cocaine, the ability of mild footshock stress (Wang et al, 2005; Wang et al, 2007) and drug-associated cues (Wise et al, 2008; You et al, 2007) to activate the dopamine system in animals with self-administration experience are mediated by experience-dependent glutamatergic input to the VTA. Moreover, experience with opiates, cocaine, amphetamine, nicotine, ethanol, and stress each share the ability to modify glutamate signaling onto dopamine neurons (Saal et al, 2003).
Thus, glutamate might be expected to play a role in the activation of the dopamine system by contextual and situational stimuli that predict heroin availability and by the mild footshock stress that reinstates heroin seeking in animals with a history of heroin self-administration. The aim of this study was to examine the role of VTA glutamate in opiate-seeking behavior. Here, we collected VTA dialysates during heroin self-administration, extinction, and footshock-induced reinstatement of the extinguished self-administration habit. Dialysates were assayed for glutamate and dopamine and the importance of VTA glutamate for extinction and reinstatement were tested by infusing a glutamate antagonist into the VTA reverse dialysis.
MATERIALS AND METHODS
Subjects
Thirty 350–400 g male Long–Evans rats (Charles River, Raleigh, NC) were used. They were housed individually under a reverse light/dark cycle (light on from 2000, h to 0800 h) with ad libitum access to food and water. They were allowed to acclimate to the new environment for at least 7 days before surgery. Experimental procedures followed the Guide for the Care and Use of Laboratory Animals published by National Institutes of Health and were approved by the local Animal Care and Use Committee.
Surgery
The rats were implanted under pentobarbital (30 mg/kg, i.p.) and chloral hydrate (140 mg/kg, i.p.) anesthesia with intravenous catheters and bilateral guide cannulae for microdialysis probes (CMA/Microdialysis, North Chelmsford, MA). The coordinates for VTA guide cannulae were: 5.6 mm posterior to bregma, 2.2 mm lateral at an angle of 12° toward the midline, and 6.7 mm ventral to the skull surface. The cannulae were anchored to the skull with stainless-steel screws and dental cement. In each animal, an intravenous SILASTIC catheter (Dow Corning, Midland, MI) was inserted into the right external jugular vein. The catheter was fed subcutaneously to exit at the back of the skull, where it was fixed to the head assembly with dental cement. Each rat was given a prophylactic subcutaneous injection of 0.25 ml of 2.27% enrolfloxacine (Baytril) daily for 3 days. The catheters were flushed daily with 0.05 ml of gentamicin (4 mg/ml in sterile saline) and 0.1 ml of heparinized saline (10 U/ml in sterile saline) before testing.
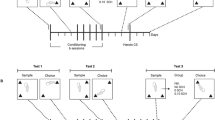
Self-Administration Training and Testing
After recovery from surgery, each rat’s catheter was connected by polyethylene tubing, protected by a metal spring, through a fluid swivel to a syringe in a microprocessor controlled syringe pump (Razel Scientific Instruments, Stamford, CT). The animal was placed in an operant chamber equipped with two levers, one fixed and one retractable, 9 cm above the chamber floor. Each training session was initiated by illumination of the animal’s 12 W house light and insertion of the response lever into the chamber. Each rat was trained to press the retractable lever (designated the ‘active’ lever) for intravenous heroin (0.1 mg/kg per injection, delivered in a volume of 0.28 ml over 28 s) on a fixed ratio-1 schedule of reinforcement; a white cue light above the lever was lighted for the duration of each injection. Lever presses during infusions or on the ‘inactive’ lever were recorded but had no scheduled consequences. The animals were trained for 10–15 days in daily 4 h sessions; within this period, each animal met the training criteria of sustained responding throughout the 4 h sessions with <20% variation in intake over the last 3 days. Following successful training, the animals were divided into four groups for subsequent testing; mean heroin intakes for the last three training days in each of the four groups were 1.233, 1.172, 1.161, and 1.283 g/kg/session.
Extinction Sessions
Following training, all rats were subjected to extinction testing, in which the testing schedule was not changed but nonrewarding saline was substituted for rewarding heroin. Three groups (n=6 each) were tested only once in extinction conditions; one group was tested in extinction conditions for 21–28 daily sessions, until their responding dropped to <15 responses for three consecutive sessions. This group was subsequently tested in a footshock-induced reinstatement paradigm (see below).
Response rates, glutamate levels, and dopamine levels were determined in the three groups, each given a single extinction test following the last training day. Here, the sessions were the same as during training except for the substitution of nonrewarding saline in place of rewarding heroin. These animals were used to determine VTA glutamate and dopamine fluctuations and to determine the effects of glutamate receptor blockade on extinction responding. The ionotropic glutamate receptor antagonist kynurenic acid (Kyn) was added to the perfusion medium during the baseline period, 60 min before initiation of the extinction session, and was continued throughout the session; one group received 0.1 mM Kyn through the dialysate whereas a second group received 1.0 mM Kyn; the vehicle, artificial cerebrospinal fluid (aCSF), was in the perfusate of the third group. Microdialysis samples were taken before, during, and after this self-administration session, as described below. The group receiving aCSF in the dialysate was the group dialyzed the day before in the final heroin self-administration session.
The animals in the fourth group did not undergo dialysis testing at this time; they were simply given continued extinction sessions for 21–28 days, until their response rates dropped to <15 responses per 4-h session. This group was then tested under microdialysis conditions for footshock-induced reinstatement of heroin seeking.
Footshock-Induced Reinstatement
After completion of the extinction phase, the remaining group (n=6) was used to determine the effect of mild footshock stress on lever pressing and on VTA glutamate and dopamine levels as in our previous studies with cocaine self-administration (Wang et al, 2005; You et al, 2007). Sensitivity to footshock was determined the evening before reinstatement testing; shock intensity (0.3–0.6 mA) was set below each animal’s freezing threshold (0.3–0.6 mA).
On the following 2 days, each animal was tested in each of the four conditions: shock vs no shock and Kyn (1.0 mM) vs no Kyn. Two tests were given each day; the shock and Kyn conditions were counterbalanced across days. The animals were given a 10-min series of unsignaled half-second trains of mild footshock at unpredictable 10–70 s intervals. For tests in which Kyn was given, it was added to the perfusion medium 50 min before the footshock period (during the period where baseline microdialysis samples were collected) and was maintained throughout. At the end of the footshock period, the house light was illuminated and the response lever was inserted into the chamber. As in the previous extinction testing, responses resulted in saline injections; lever presses and saline injections were recorded for 2 h. Microdialysis samples were taken before, during, and after the session, as described below.
Microdialysis
The evening before each test day, the blockers were removed from their guide cannulae and VTA microdialysis probes (CMA/12 14/02) were inserted and fixed in position. The probes were connected to the microdialysis pump (CMA/100) through the two channels of a three-channel feed-through swivel (Scipro, Sanborn, NY) with fluorinated ethylenepropylene tubing. The third channel was used to connect the intravenous catheter to its injection pump. Each rat was infused overnight with aCSF (in mM: 148 NaCl, 2.7 KCl, 1.2 CaCl2, and 0.8 MgCl2, pH 7.4) at a flow rate of 0.4 μl/min. The following morning, the flow rate was increased to 2 μl/min for a 30-min equilibration period and four 10-min baseline samples were taken before the scheduled test. Each test was initiated by insertion of the response lever into the chamber along with illumination of the animal’s 12 W house light. Microdialysis samples from one hemisphere were analyzed for glutamate and samples from the other hemisphere were analyzed for dopamine. For the animals from which microdialysis samples were taken on 2 days (the last day of self-administration and the following day in extinction conditions), the probes were left in place and perfused overnight at 0.4 μl/min between the two tests.
Biochemical Analysis of Microdialysis Samples
Glutamate concentrations were determined by HPLC. An ESA 582 pump (ESA, Chelmsford, MA), a CMA/260 degasser, a CMA/200 refrigerated microsampler, a phase II ODS column (3 μm particle size, 3.2 × 100 mm; Bioanalytical Systems, West Lafayette, IN), a CMA/280 fluorescence detector, and an ESA model 501 data station were used. The CMA/280 is a fixed-wavelength fluorescence detector operating at a maximal excitation of 330–365 nm and emission of 440–530 nm. We performed precolumn derivation of glutamate with an o-phthalaldehyde/mercaptoethanol reagent (in M: 0.4 borate, 0.04 phthalaldehyde, and 0.4 2-mercaptoethanol, pH 10.4). Briefly, 10 μl of the reagent was added to and mixed with the samples by the microsampler. After a 60 s reaction period at 6 °C in the microsampler, 20 μl of the mixture was injected onto the column. The elution of glutamate was achieved with a mobile phase consisting of 0.15 M sodium acetate, 10% methanol, and 1.5% tetrahydrofuran at a flow rate of 0.6 ml/min. After the appearance of the glutamate peak on the chromatogram, an injection of 20 μl of 100% methanol was made by the microsampler to accelerate the elution of the residuals on the column. The detection limit was 0.2 pmol/injection.
Dopamine was measured with HPLC coupled to an ESA Coulochem II Detector (model 5200) with a dual-electrode microdialysis cell and an ESA model 501 data station. Samples were manually injected onto the column (3 μm particle size, 3 × 150 mm; Analytical MD-150; ESA). The mobile phase for dopamine separation consisted of 75 mM NaH2PO4, 1.5 mM N-1-octanesulfonic acid, 10 μM EDTA, and 8% acetonitrile, pH 3.0, adjusted with H3PO4. Dopamine was quantified on both reducing (−250 mV) and oxidizing (350 mV) electrodes. The limit of detection for dopamine was ∼5 fmol/injection.
Basal glutamate and dopamine levels did not differ significantly either between groups or across days in the reinstatement test (Table 1). Levels are expressed as percent of baseline for ease of comparison in the figures.
Drugs
Heroin and anesthetics were obtained from the NIDA pharmacy. Kyn was purchased from Sigma. Kyn was dissolved first with a small aliquot of 5 N NaOH and diluted with aCSF to the final concentration; the solution was adjusted to pH 7.4 before use.
Histology
After microdialysis, the rats were decapitated under anesthesia and their brains were removed and fixed in 10% formalin. The fixed brains were frozen and 50 μm coronal sections were taken; probe location was determined under low magnification after wetting the tissue to differentiate fibers from cell masses. Two animals (from different groups) were excluded from the analysis because of probe locations outside the VTA. Four animals failed to complete the training phase: two because they bit through their microdialysis lines; one because it dislodged its headpiece; and one because it died during training. Confirmed probe placements are shown in Figure 4.
Statistics
To determine the effect of heroin self-administration on levels of each neurotransmitter (Figure 1), we used repeated measures ANOVAs with Fisher’s LSD post hoc comparisons. To determine the effect of lever insertion on behavior in extinction (Figure 2a) we used one-way ANOVA. To determine the effects on neurotransmitter levels (Figure 2b and c) we used two-way ANOVA with repeated measures comparing transmitter levels for 30 min before lever insertion with levels for 30 min after lever insertion. To determine the effect of lever insertion on behavior in the reinstatement tests (Figure 3a) we used a 2 × 2 repeated measures ANOVA with SHOCK (vs no shock) and DRUG (Kyn vs no Kyn) as factors. To determine the effects on neurotransmitter levels (Figure 3b and c) we again compared transmitter levels for 30 min before vs 30 min after lever insertion, using three-way repeated measures ANOVA with TIME (before vs after insertion), SHOCK, and DRUG as factors.
Mean dopamine and glutamate levels in 10-min microdialysis samples taken before, during, and after the final heroin self-administration day in animals that had received 14 previous days of self-administration training. Before the microdialysis tests, animals were housed and dialyzed at low rates in the test chamber overnight. On the test morning, the infusion rate was increased and baseline samples taken; then (at t=0), the response lever was inserted into the chamber and heroin became available for 4 h. The times of lever insertion and retraction are indicated by dashed vertical gray lines. Initial lever presses usually occurred within 3 min of lever insertion; the animals pressed at ∼20 min intervals thereafter, receiving ∼300 ng/h of heroin, by the end of the session. Traces a–f below the graph show the temporal pattern of responding for each animal. Vertical hash marks on each timeline indicate the times (relative to the graph above) of each earned injection for each animal.
The effects of VTA ionotropic glutamate receptor blockade on response rates (a) and glutamate (b) and dopamine (c) levels during a single initial extinction test that followed 2 weeks of self-administration training. Insertion of the response lever was at time=0, marked by the dashed vertical gray lines. Three independent groups were tested: two receiving the ionotropic glutamate receptor blocker kynurenic acid (Kyn: 0.1 or 1.0 mM by reverse dialysis), and one receiving only the Kyn vehicle (aCSF). The response lever was inserted at t=0, indicated by dashed vertical gray lines. Kyn significantly attenuated responding and dopamine but not glutamate levels during extinction.
The effects of mild footshock stress and Kyn (1.0 nM) on responding (a) and VTA glutamate (b) and dopamine levels (c) after 2 weeks of self-administration training, followed by 3 weeks of extinction testing. Response levers were inserted into the testing chambers at the end of the 10-min period of intermittent footshock.
Probe placements are indicated by the straight, black, angled vertical line segments at the base of each brain drawing. The number of lines is less than the number of animals because of overlap in the placements. Drawings were adapted from the atlas of Paxinos and Watson (1998); the numbers on the right side of the drawings indicate the distance (in millimeters) posterior to bregma.
RESULTS
Heroin Self-Administration
Dopamine levels were elevated significantly during heroin self-administration (Figure 1) as reflected in a main effect of time (F38, 190=5.91, P<0.0001). The post hoc comparisons showed that dopamine levels remained significantly elevated from the first to the last sample within the self-administration period and were no longer significantly elevated in the first or any subsequent sample following the last heroin injection. There was no significant effect of time on glutamate levels (F38, 190=1.11, P=0.31).
Extinction Test
Responding in extinction was inhibited by VTA perfusion of the glutamate antagonist Kyn (Figure 2a). This was reflected in a significant effect of Group (F2, 15=73.63; P<0.0001), with the aCSF group responding significantly more than either of the Kyn groups (t10=9.27, P<0.0001; t10=11.42, P<0.0001). The groups given Kyn did not differ significantly from each other (t10=2.14, P=0.048, NS, with Bonferroni correction for multiple comparisons).
VTA glutamate release was significantly elevated during initial 30 min of extinction responding (Figure 2b) as reflected in a main effect of Time (F1, 15=42.38; P<0.0001). There was no significant effect of Group.
VTA dopamine release was differentially elevated during initial extinction responding (Figure 2c) as reflected in a Group × Time interaction (F2, 15=4.98, P<0.03). The post hoc comparison confirmed that dopamine levels were significantly elevated in the aCSF group (t5=2.94, P=0.032).
Reinstatement Test
Footshock stress reinstated lever pressing only in the control group that was treated with aCSF (Figure 3a). This was reflected in an interaction between Shock and Drug (F1, 5=35.75, P<0.002), with responding in the footshock-alone condition significantly greater than responding in the footshock/Kyn condition (t5=7.97, P<0.0005).
Footshock stress elevated glutamate levels (Figure 3b) in both Kyn-treated and aCSF-treated animals (Shock × Time interaction, F1, 5=117.95, P<0.0001); there was no significant effect of Kyn in the absence of footshock.
Footshock stress elevated dopamine levels in only the control group that was treated with aCSF. This was reflected in a Time × Drug interaction (F1, 5=117.95, P<0.0001). A planned contrast test showed the dopamine levels in the aCSF condition to be elevated more than in each of the others (F1=110.75, P<0.0001).
DISCUSSION
These findings implicate VTA glutamatergic signaling in the effects of reward-predictive cues and footshock stress in heroin seeking, indicating similar roles to those previously reported in parallel studies of cocaine seeking (Wang et al, 2005; You et al, 2007). More importantly, they suggest that heroin experience causes the same neuroadaptations that establish glutamate responsiveness to predictive cues and mild footshock stress. It is perhaps not surprising that the same conditioned glutamate responses develop to the cues associated with an unanticipated extinction session. However, it is somewhat surprising that drugs with such different receptor-mediated actions should each establish glutamatergic responsiveness to footshock. That both cocaine and heroin should establish experience-dependent glutamate release in response to footshock is consistent, however, with the fact that they each establish experience-dependent potentiation of responsiveness of dopaminergic neurons to glutamate stimulation (Saal et al, 2003).
Stimulation of group II metabotropic glutamate autoreceptors in the VTA—a treatment presumed to attenuate evoked glutamate release (Schoepp, 2001)—attenuates reinstatement of heroin seeking by contextual cues (the operant chamber) previously associated with heroin self-administration (Bossert et al, 2004). In the present case, responding was triggered and temporarily maintained under extinction conditions by more punctate cues: a discriminative stimulus—insertion into the test chamber of the active lever that had previously indicated drug availability—and a conditioned reinforcing stimulus—the cue light illumination that had previously signaled completion of the required instrumental response. Although the two kinds of cues were not differentiated in this study, it is known that each contributes to drug-seeking response habits (Grimm et al, 2001). Here, insertion of the response lever and cue delivery, and responding during the first minutes of the extinction—or perhaps some combination of the three—caused glutamate and dopamine release in the VTA, and blocking VTA glutamate receptors attenuated the dopamine response and the behaviors. The source of the dopamine release is the dopaminergic neurons in the region; these neurons release dopamine from their dendrites (Geffen et al, 1976) when activated (Legault et al, 1999, 2000, 2001). Rats exposed to the conditions of self-administration but offered saline rather than a rewarding drug show no significant glutamate or dopamine response to the lever insertion or cue light presentation we used in similar experiments with cocaine (You et al, 2007). Thus, heroin-predictive cues—like cocaine-predictive cues and footshock stress—appear to cause glutamate release in heroin-experienced animals, and this glutamate release, in turn, appears to activate VTA dopamine neurons and triggers initiation of the established response habit. In this regard, the mechanisms of heroin seeking and cocaine seeking are similar.
The surprising difference between heroin seeking and cocaine seeking is that whereas VTA glutamate and dopamine are elevated when animals begin to respond and receive the expected cocaine, VTA glutamate was not elevated during the first few minutes of responding and actually receiving heroin (Figure 1). Despite the presence of the same heroin-predictive cues when heroin is actually earned, VTA glutamate was not elevated and dopamine was elevated to a little more than 200% of baseline, about half the elevation in VTA dopamine that is seen when cocaine is earned (You et al, 2008). Given that the cues associated with the initial minutes of heroin self-administration are the same as those in the initial minutes of extinction responding, it is clearly a unique pharmacological effect of heroin that is responsible for the lack of glutamate release in the early minutes of heroin self-administration. Although many of the differences between animals self-administering cocaine and heroin (Badiani et al, 2011) are due to side effects unrelated to the rewarding actions of the drugs, this would appear to be one of the important differences between heroin and cocaine self-administration that involves the reward system itself.
It is tempting to attribute the difference between the glutamate response to the initiation of a heroin self-administration session and the glutamate response to the initiation of an extinction session to differences in some aspect of reward expectancy. The dopamine system is known to be activated by unexpected rewards and not by fully expected rewards (Schultz, 1998); inasmuch as glutamate represents one of the two expectancy-related inputs to the VTA (You et al, 2007; You et al, 2008), it might be suggested that there is no glutamate response when the heroin reward is given because our animals are well trained and the heroin reward is fully expected. This suggestion does not fit well with the fact that similar glutamate responses are seen in initiation of cocaine self-administration and in the early minutes of the first extinction trial in cocaine studies (You et al, 2007). Thus, it seems more likely that the lack of elevated glutamate release in the early minutes (sampled at 10-min intervals in this study) of heroin self-administration is because of some pharmacological effect of the earned heroin. The primary effects of opioids on the dopamine system have been thought to result from the inhibition of GABAergic neurons that normally hold their dopaminergic neighbors under inhibitory control (Johnson and North, 1992). However, more recent studies show that μ-opioid agonists also exert presynaptic control over glutamate inputs to the VTA, reducing glutamate currents evoked in dopamine neurons (Bonci and Malenka, 1999; Manzoni and Williams, 1999; Margolis et al, 2005). There may be an immediate increase in glutamate level before the first earned injection that is masked, in our 10-min dialysis samples, by the net effect of heroin itself when it arrives a few seconds after the predictive stimuli. Studies utilizing the rapid response of voltammetry to monitor glutamate levels in the first seconds of self-administration experiments may help to clarify this issue.
References
Anderson SM, Bari AA, Pierce RC (2003). Administration of the D1-like dopamine receptor antagonist SCH-23390 into the medial nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug-seeking behavior in rats. Psychopharmacology 168: 132–138.
Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011). Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12: 685–700.
Bonci A, Malenka RC (1999). Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci 19: 3723–3730.
Bossert JM, Liu SY, Lu L, Shaham Y (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24: 10726–10730.
Bozarth MA, Wise RA (1981). Heroin reward is dependent on a dopaminergic substrate. Life Sci 29: 1881–1886.
Carlezon WA, Wise RA (1996). Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate lateral hypothalamic brain stimulation reward. Psychopharmacology 128: 413–420.
Devine DP, Leone P, Pocock D, Wise RA (1993). Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther 266: 1236–1246.
Devine DP, Wise RA (1994). Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci 14: 1978–1984.
Geffen LB, Jessell TM, Cuello AC, Iversen LL (1976). Release of dopamine from dendrites in rat substantia nigra. Nature 260: 258–260.
Geisler S, Wise RA (2008). Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci 19: 227–244.
Geisler S, Zahm DS (2005). Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol 490: 270–294.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Incubation of cocaine craving after withdrawal. Nature 412: 141–142.
Johnson SW, North RA (1992). Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neuroscience 12: 483–488.
Legault M, Rompré P-P, Wise RA (2000). Chemical stimulation of the ventral hippocmpus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci 20: 1635–1642.
Legault M, Wise RA (1999). Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse 31: 241–249.
Legault M, Wise RA (2001). Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci 13: 819–828.
Manzoni OJ, Williams JT (1999). Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci 19: 6629–6636.
Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2005). Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol 93: 3086–3093.
Matsui A, Williams JT (2011). Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci 31: 17729–17735.
Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates, 4th edn. Academic Press: San Diego, CA.
Phillips AG, LePiane FG (1980). Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav 12: 965–968.
Saal D, Dong Y, Bonci A, Malenka RC (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582.
Schmidt HD, Pierce RC (2006). Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience 142: 451–461.
Schoepp DD (2001). Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299: 12–20.
Schultz W (1998). Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27.
Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A (2008). Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci 28: 8406–8416.
Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB (2005). Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25: 5389–5396.
Wang B, You ZB, Rice KC, Wise RA (2007). Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology 193: 283–294.
Wise RA (2008). Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14: 169–183.
Wise RA, Bozarth MA (1981). Brain substrates for reinforcement and drug self-administration. Prog Neuropsychopharmacol 5: 467–474.
Wise RA, Murray A, Bozarth MA (1990). Bromocriptine self-administration and bromocriptine-reinstatement of cocaine-trained and heroin-trained lever pressing in rats. Psychopharmacology 100: 355–360.
Wise RA, Rompré P-P (1989). Brain dopamine and reward. Ann Rev Psychol 40: 191–225.
Wise RA, Wang B, You ZB (2008). Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS One 3: e2846.
Xi ZX, Stein EA (2002). Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology 164: 144–150.
You ZB, Wang B, Zitzman D, Azari S, Wise RA (2007). A role for conditioned ventral tegmental glutamate release in cocaine seeking. J Neurosci 27: 10546–10555.
You ZB, Wang B, Zitzman D, Wise RA (2008). Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci 28: 9021–9029.
Zangen A, Ikemo S, Zadina JE, Wise RA (2002). Rewarding and psychomotor stimulant effects of endomorphin-1: anterior-posterior differences within the ventral tegmental area and lack of effect in nucleus accumbens. J Neurosci 22: 7225–7233.
Acknowledgements
This study was supported by the Intramural Research Program, National Institute on Drug Abuse.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, B., You, ZB. & Wise, R. Heroin Self-Administration Experience Establishes Control of Ventral Tegmental Glutamate Release by Stress and Environmental Stimuli. Neuropsychopharmacol 37, 2863–2869 (2012). https://doi.org/10.1038/npp.2012.167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.167
Keywords
This article is cited by
-
Relapse to opioid seeking in rat models: behavior, pharmacology and circuits
Neuropsychopharmacology (2019)
-
Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress
Neuropsychopharmacology (2016)
-
Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration
Molecular Psychiatry (2016)
-
N-methyl-d-aspartate receptors in the ventral tegmental area mediate the excitatory influence of Pavlovian stimuli on instrumental performance
Brain Structure and Function (2016)
-
The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research
Psychopharmacology (2013)