Abstract
Background/Objectives:
Determining long-term trends in tumor biomarker expression is essential for understanding aspects of tumor biology amenable to change. Limiting the availability of such data, currently used assays for biomarkers are relatively new. For example, assays for the estrogen receptor (ER), which are the oldest, extend back only to the 1970s.
Methods:
To extend scant knowledge about the feasibility of obtaining long-term data on tumor biomarkers, we randomly selected 60 breast cancer cases (10 per decade) diagnosed between 1947–2009 among women members of the Kaiser Permanente Northern California health plan to obtain and analyze their formalin-fixed paraffin-embedded (FFPE) tumor specimens. For each tumor specimen, we created duplicate tissue microarrays for analysis.
Results:
We located tumor blocks and pathology reports for 50 of the 60 cases (83%), from which we randomly sampled 5 cases per decade for biomarker analysis (n=30). All 30 cases displayed excellent morphology and exhibited biomarkers compatible with histologic type and grade. Test–retest reliability was also excellent: 100% for ER; 97% for human epidermal growth factor receptor 2 and epidermal growth factor receptor; 93% for progesterone receptor and cytokeratin 5/6; and 90% for Ki67 and molecular phenotype; the kappa statistic was excellent (>0.9) for 4 of the 7 biomarkers, strong (0.6–0.8) for 2, and fair for only 1 (owing to low prevalence).
Conclusions:
These results indicate immunostaining for biomarkers commonly used to evaluate breast cancer biology and assign surrogate molecular phenotypes can reliably be employed on archival FFPE specimens up to 60 years old.
Similar content being viewed by others
Introduction
Determining long-term trends in tumor biomarkers is crucial for understanding what aspects of tumor biology are amenable to change. Evidence of long-term trends critically complements cross-sectional comparisons, across geographical regions or social groups, because only long-term data can detect the impact of changing exogenous exposures.1 For example, the recent rise and fall in breast cancer incidence in many countries, linked to the rise and fall of postmenopausal hormone therapy use2–9 was paralleled by a rise and fall in the incidence of estrogen receptor positive (ER+) tumors.3,4 Of note, although breast cancers tumors are generally more often ER+ among US white compared with black women,10 the US white/black odds ratio for ER+ breast tumors nevertheless exhibited a parallel rise and fall during this same time period, a pattern likely attributable to changes in hormone therapy use.11
Scant knowledge, however, exists about the feasibility of locating and analyzing decades old, population-based archival formalin-fixed paraffin-embedded (FFPE) tumor specimens. Contributing to the lack of such historical data is the relatively recent development of most currently used assays: in the case of breast cancer, for example, one of the first such assays, for ER, became available only in the 1970s,12 and characterization of molecular phenotypes is an innovation of the 21st century CE.13 We accordingly conducted a novel feasibility study, including assessment of test–retest reliability, for a series of breast cancer cases spanning 6 decades (1947–2009). Favorable results would enhance interpretation of prior studies that have employed biomarker immunostains on old FFPE specimens, e.g., 30–40 years old when analyzed,14,15 as well as encourage new research.
Materials and Methods
For our study, we analyzed FFPE specimens obtained from women diagnosed with invasive breast cancer who were members of Kaiser Permanente, Northern California (KPNC; institutional review board approval: Harvard School of Public Health/#CR-20929–02; KPNC/#CN-13LHabe-03-H). KPNC is an integrated healthcare delivery system established in the 1940s16 and whose cancer registry dates back to 1947.17 Since its inception as a health plan for workers employed in World War II shipyards, KPNC’s membership has ranged from working class to professional and has mirrored the well-known diversity of the San Francisco Bay Area and Central Valley, comprised of white, black, Hispanic, Asian and Pacific Islander, and American Indian populations.17,18
Among the 60,904 breast cancer cases diagnosed between 1947 and 2009, 7,150 met our feasibility study’s eligibility criteria: 50–64 years old at diagnosis and invasive tumor⩾1 cm. We randomly selected 10 eligible cases per each of the 6 time periods (hereafter referred to as decades: 1947–1959; 1960–1969;…; 2000–2009), and used information available as of 1987 to restrict sampling to cases with lymph node positive tumors. Our rationale was to maximize the chance that biomarkers would be positive or credibly negative, given that advanced cases are more likely to be positive for human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), high Ki67, ER−, and PR−.13,19 Thus, had the specimens included only early stage cancer, it would be less clear if negative test results could be interpreted as truly negative—versus falsely negative—because the assay was not sensitive to biomarker expression in the older specimens. Among the 50 cases located with eligible blocks containing tumor (as described below), and in accord with our a priori power calculations, we selected a random sample of 5 cases per decade (total n=30) for biomarker immunohistochemical analysis.
To conduct the assays, we first created tissue microarrays (TMAs) in duplicate, each employing 3, 0.6-mm cores per specimen. We assessed antigen preservation by using a cytokeratin (CK) “cocktail” immunostain (consisting of antibodies AE1/AE3 and Cam5.2, which together recognize a broad spectrum of CKs), and also performed immunostains for biomarkers routinely used to assess breast cancer biology and assign molecular phenotype based on surrogate markers13,19–22: ER, progesterone receptor (PR), HER2, CK 5/6, EGFR, and Ki67. The Ki67 stains were quantitated by computer-assisted image analysis;23 for each case, the score equaled the average percentage of positive cells per core. Review of the biomarkers was blinded to tumor histologic type and grade, and review of each set of TMA results was independent and blinded to the other. For each TMA, if a tumor marker was scored as positive for 1 or more cores, it received an overall rating of positive for that marker.
Results
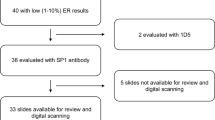
Among the random sample of 60 selected cases, we located pathology reports for 55 cases (92%), of which 50 (83% of the 60) had blocks that contained tumor tissue. Among the random sample of 5 cases per decade selected from these 50 cases, notably all 30 cases (100%) displayed excellent morphology and the CK cocktail staining results (Table 1) indicated antigen integrity was preserved for all but 2 of the cases (1 from the 1950s, 1 from the 1960s). Test–retest reliability was likewise excellent (Table 1): 100% for ER, 97% for HER2 and EGFR, 93% for PR and CK 5/6, and 90% for Ki67 (scored as <14% vs. ⩾14%19). The kappa statistic (taking into account chance agreement;24 Table 1) was excellent (>0.9) for 4 of the 7 biomarkers, moderate-to-strong (0.6–0.8) for 2, and fair for 1 (0.346 for Ki67 as a dichotomous variable). On the basis of the biomarker results, concordance for molecular phenotype was 90% (kappa >0.7): classification of 15 cases was concordant for Luminal A, 3 for Luminal B, 3 for basal-like, 3 for HER2, and 2 for “unclassified”; only 3 cases were discordant for classification as either Luminal A or B.
Assay results indicated that ~80% of tumors were ER+ (i.e., 80% for all decades except for the 1960s, for which 3/5 (60%) of the cases were ER+), all ER− tumors were grade 3, and virtually all ER+ tumors were grade 1 or grade 2. For PR, 60% of tumors were PR+ and all but one of the PR− tumors was grade 3 (the exception was grade 1). In addition, most tumors were negative for: HER2 (60–80% negative); CK 5/6 (87% negative); and EGFR (93% negative); Ki67 was <14% for 86–90% of the cases.
Discussion
Considered together, our findings provide promising evidence for investigators seeking to conduct analyses of historical trends in tumor characteristics. First, perhaps unique to KPNC, we were able to locate pathology reports and informative tumor blocks for 83% of cases diagnosed between 1947 and 2009, with no discernible difference in specimen retrieval by decade. Second, more generalizably, we demonstrated that, among a random sample of breast cancer tumor specimens spanning this time period, current immunostaining methods for biomarkers commonly used to evaluate breast cancer biology and assign molecular subtype13,19–22 yielded plausible results for both old and recent specimens,13,19 with excellent test–retest reliability. Of note, the lower test–retest reliability (93%) observed for both PR and CK 5/6 is consistent with prior studies indicating that expression of these biomarkers may demonstrate intratumor heterogeneity,21,22 regardless of the assay used. The high concordance (90%) but low kappa (0.346) for Ki67 reflects the low prevalence of high values.24
In summary, our study provides novel evidence that current immunostain techniques can feasibly and reliably be used on old FFPE specimens, dating back 60 years. It accordingly suggests that prior14,15 and future studies employing immunostains to characterize long-term trends in tumor biomarker expression at the population level can yield credible results.
References
Krieger N, Chen JT, Kosheleva A, Waterman PD . Shrinking, widening, reversing, and stagnating trends in US socioeconomic inequities in cancer mortality: 1960-2006. Cancer Causes Control 2012; 23: 297–319.
Krieger N, Chen JT, Waterman PD . Decline in US breast cancer rates after the women’s health initiative: socioeconomic and racial/ethnic differentials. Am J Public Health 2010; 100: S132–S139, Erratum in Am J Public Health. 2010; 100: 972.
Krieger N . Hormone therapy and the rise and perhaps fall of US breast cancer incidence rates: critical reflections. Int J Epidemiol 2008; 37: 627–637.
Glass AG, Lacey JV Jr, Carreon JD, Hoover RN . Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst 2007; 99: 1152–1161.
Clarke CA, Glaser SL . Declines in breast cancer after the WHI: apparent impact of hormone therapy. Cancer Causes Control 2007; 18: 847–852.
Canfell K, Banks E, Moa AM, Beral V . Decrease in breast cancer incidence following a rapid fall in the use of hormone replacement therapy in Australia. Med J Aust 2008; 188: 641–644.
Neutel CI, Morrison H . Could recent decreases in breast cancer incidence really be due to lower HRT use? Trends in attributable risk for modifiable breast cancer risk factors in Canadian women. Can J Public Health 2010; 101: 405–409.
Katalinic A, Lemmer A, Zawinell A, Rawal R, Waldmann A . Trends in hormone therapy and breast cancer incidence—results from the German Network of Cancer Registries. Pathobiology 2009; 76: 90–97.
Parkin D . Is the recent fall in incidence of postmenopausal breast cancer in the UK related to changes in the use of hormone replacement therapy. Eur J Cancer 2009; 45: 1649–1653.
Brawley OW . Health disparities in breast cancer. Obstet Gynecol Clin North Am 2013; 40: 513–523.
Krieger N, Chen JT, Waterman PD . Temporal trends in the black/white breast cancer case ratio for estrogen receptor status: disparities are historically contingent, not innate. Cancer Causes Control 2011; 22: 511–514.
McGuire WL . Current status of estrogen receptors in human breast cancer. Cancer 1975; 36: 638–644.
Goldhirsch A, Winer EP, Coates AS, Gelberr RD, Piccart-Gebhart M, Thülimann B et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24: 2206–2223.
Krieger N, Van Den Eeden SK, Zava D, Okamoto A . Race/ethnicity, social class, and prevalence of breast cancer molecular prognostic biomarkers: a study of white, black, and Asian women in the San Francisco Bay Area. Ethn Dis 1997; 7: 137–149.
Engstrøm MJ, Opdahl S, Hagen AI, Romunstad PR, Akslen LA, Haugen OA et al. Molecular subtypes, histopathological grade, and survival in a historic cohort of breast cancer patients. Breast Cancer Res Treat 2013; 140: 463–473.
Hendricks RL . A Model for National Health Care: The History of Kaiser Permanente. Rutgers University Press: New Brunswick, NJ, USA, 1993.
Kaiser Permanente Division of Research. Division of Research Maintained Data Sources: KP Northern California Cancer Registry. Available at http://www.dor.kaiser.org/external/DORExternal/research/ctsi/data_sources.aspx?id=2668#disease (accessed on 14 September 2015).
Gordon N . Kaiser Permanente Division of Research. What the Member Health Surveys Project Tells Us about the Kaiser Permanent Northern California Adult Membership: Demographics, IT Access, Behavioral/Lifestyle Risks, and Health: Trends, Race-Ethnic Differences, and Variation across Service Populations 2008, Available at http://www.dor.kaiser.org/external/uploadedFiles/content/research/mhs/Other_Reports/mhs_project_trends-1993-2005.pdf (accessed on 14 September 2015).
Zaha DC . Significance of immunohistochemistry in breast cancer. World J Clin Oncol 2014; 5: 382–392.
Ades F, Zardavas D, Bosovic-Spasojevic I, Pugliano L, Fumagalli D, Azambuja ED et al. Luminal B breast cancer: molecular characterization, clinicial management, and future perspectives. J Clin Oncol 2014; 32: 2794–2803.
Sighoko D, Liu J, Hou N, Gustafson P, Huo D . Discordance in hormone receptor status among primary, metastatic, and second primary breast cancers: biological difference or misclassification? Oncologist 2014; 19: 592–601.
Badve S, Dabbs DJ, Schnitt SJ, Baehner F, Decker T, Eusebi V et al. Basal-like and triple negative breast cancer: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol 2011; 24: 157–167.
Definiens Tissue Studio, version 3.6.1. Available at http://www.definiens.com/solutions-overview/product-list/definiens-tissue-studio.html (accessed on 14 September 2015).
Viera AJ, Garrett JM . Understanding interobserver agreement: the kappa statistic. Fam Med 2005; 37: 360–363.
Acknowledgements
We acknowledge, with permission (email: 8 February 2015), Dr Andrew Beck (Director, Molecular Epidemiology Research Laboratory, Pathology, Beth Israel Deaconess Medical Center, Boston, MA, USA) and Dr Laleh Montaser-Kouhsari (Post-doctoral Researcher, Pathology, Beth Israel Deaconess Medical Center, Boston, MA, USA), for their assistance with the computer-assisted image analysis for the Ki67 assay results.
FUNDING
This work was supported by the National Cancer Institute (NCI) at the National Institutes of Health (NIH), grant number 1R21CA166115-01A1 (“Long-term trends in breast cancer tumor profiles & disparities”), awarded to NK. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article; and decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
NK conceived the study, designed and supervised the analyses, led interpretation of the results, and wrote the first draft of the article. All authors designed the study and wrote the article. LAH led the work in identifying the eligible study participants and obtaining the tumor specimens, assisted by NA, and both contributed to interpreting results. SJS led the work on tissue microarray construction and evaluation of biomarker expression, assisted by MS, and both contributed to interpreting the results. PDW and LA assisted with study logistics and contributed to interpreting the results. LE-L contributed to interpreting results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Krieger, N., Habel, L., Waterman, P. et al. Analyzing historical trends in breast cancer biomarker expression: a feasibility study (1947–2009). npj Breast Cancer 1, 15016 (2015). https://doi.org/10.1038/npjbcancer.2015.16
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/npjbcancer.2015.16
This article is cited by
-
Feasibility of analyzing DNA copy number variation in breast cancer tumor specimens from 1950 to 2010: how old is too old?
Cancer Causes & Control (2018)