Abstract
The synapse specificity of long-term potentiation (LTP) ensures that no interference arises from inputs irrelevant to the memory to be encoded. In hippocampi of aged (21–28 months) mice, LTP was relayed to unstimulated synapses, blemishing its synapse specificity. Diminished levels of the K+/Cl− cotransporter KCC2 and a depolarizing GABAA receptor-mediated synaptic component following LTP were the most likely causes for the spreading of potentiation, unveiling mechanisms hindering information storage in the aged brain and identifying KCC2 as a potential target for intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Morrison, J.H. & Baxter, M.G. Nat. Rev. Neurosci. 13, 240–250 (2012).
Rosenzweig, E.S. & Barnes, C.A. Prog. Neurobiol. 69, 143–179 (2003).
Malenka, R.C. & Bear, M.F. Neuron 44, 5–21 (2004).
Kastellakis, G., Cai, D.J., Mednick, S.C., Silva, A.J. & Poirazi, P. Prog. Neurobiol. 126, 19–35 (2015).
Cox, K.J. & Adams, P.R. Front. Comput. Neurosci. 3, 11 (2009).
Cheng, Y.F. et al. Neurochem. Res. 38, 98–107 (2013).
Ris, L. & Godaux, E. Learn. Mem. 14, 185–189 (2007).
Larson, J. & Munkácsy, E. Brain Res. 1621, 38–50 (2015).
Larson, J., Lynch, G., Games, D. & Seubert, P. Brain Res. 840, 23–35 (1999).
Megías, M., Emri, Z., Freund, T.F. & Gulyás, A.I. Neuroscience 102, 527–540 (2001).
Higley, M.J. Nat. Rev. Neurosci. 15, 567–572 (2014).
Wigström, H. & Gustafsson, B. Nature 301, 603–604 (1983).
Gagnon, M. et al. Nat. Med. 19, 1524–1528 (2013).
Kaila, K., Price, T.J., Payne, J.A., Puskarjov, M. & Voipio, J. Nat. Rev. Neurosci. 15, 637–654 (2014).
Medina, I. et al. Front. Cell. Neurosci. 8, 27 (2014).
Raimondo, J.V., Kay, L., Ellender, T.J. & Akerman, C.J. Nat. Neurosci. 15, 1102–1104 (2012).
Andrásfalvy, B.K. & Mody, I. J. Physiol. (Lond.) 576, 191–196 (2006).
Doyon, N. et al. PLoS Comput. Biol. 7, e1002149 (2011).
Mohapatra, N. et al. Sci. Rep. 6, 23196 (2016).
Arosio, D. & Ratto, G.M. Front. Cell. Neurosci. 8, 258 (2014).
Bakker, A. et al. Neuron 74, 467–474 (2012).
Koh, M.T., Haberman, R.P., Foti, S., McCown, T.J. & Gallagher, M. Neuropsychopharmacol. 35, 1016–1025 (2010).
Ferando, I. & Mody, I. Front. Neural Circuits 7, 144 (2013).
Sarkar, J., Wakefield, S., MacKenzie, G., Moss, S.J. & Maguire, J. J. Neurosci. 31, 18198–18210 (2011).
Ferando, I. & Mody, I. Neuropharmacology 88, 91–98 (2015).
Preibisch, S., Saalfeld, S. & Tomancak, P. Bioinformatics 25, 1463–1465 (2009).
Acknowledgements
We thank W. Wei for performing the whole-cell recordings, A.M. Barth for help with Igor procedures, L. Ormiston and R.M. Lazaro for expert technical assistance, and all members of the Mody laboratory for comments during data presentation sessions. This research was supported by NIH-NINDS grant NS030549 (I.M.) and the Coelho Endowment to I.M. I.F. was a recipient of a postdoctoral fellowship (American Epilepsy Society Award ID 367390). Confocal laser scanning microscopy was performed at the CNSI Advanced Light Microscopy/Spectroscopy shared resource facility at UCLA, supported with funding from an NIH- NCRR shared resources grant (CJX1-443835-WS-29646) and an NSF Major Research Instrumentation grant (CHE-0722519).
Author information
Authors and Affiliations
Contributions
I.F. performed experiments and collected data. I.F., G.C.F. and I.M. conceived the study, designed the experiments, analyzed and interpreted the data, prepared the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
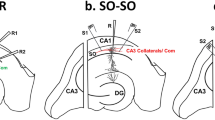
Supplementary Figure 1 Test for independence of two synaptic inputs to CA1.
(a) Illustration depicting the stimulating and recording electrode arrangements. The two pathways (S1 and S2) were stimulated with two different stimulating electrodes positioned in the str. radiatum on each side of the recording electrode. (b) Test for independence of S1 and S2. The sample traces show the testing of the two pathways before delivery of the TBS. In both young and old the stimulation of the same pathway at an interval of 50 ms (S1→S1 or S2→S2) produces paired-pulse facilitation (PPF) which is not transferred to the other pathway (S1→S2 or S2→S1), indicating input independence. Calibration bars: 0.5 mV, 5 ms.
Supplementary Figure 2 Correspondence between the ages of humans and mice for mouse ages between 3 and 30 months.
To estimate the age correspondence between human and mouse ages we used data from one review paper and one book chapter on aging in laboratory animals. Both sources compare mouse age with human age and quantify correspondences between the species at certain points during their lifetime based on a variety of factors such as sexual maturity, deterioration of locomotion and endurance, social behavior and cognitive development. Above 3 months of mouse age the correlation with human age is nearly linear (R=0.98). The plot includes data from both sources with a liner fit to estimate how human age (AH) corresponds with the age of mice (AM) over their lifespan: AH (years) = 2.4 × AM (≥ 3 months) + 11. The age range of the young (blue) and old (red) mice used in our study is indicated. The grey bar indicates the age range of the mice used in the previously published two studies (refs 6 and 7 in the manuscript) describing the lack of LTP synapse specificity.
Supplementary Figure 3 Types of LTP observed in hippocampal slices of young and old mice.
(a) Plots of the averages of fEPSP slopes recorded before and after the TBS (indicated by ▲). Based on the trajectories of the plots (see Methods for details) we identified three types of responses present in both young and old slices: 1) no LTP; 2) steady state LTP (ss LTP); and 3) rising LTP (r LTP). (b) The distribution of the three types of responses in young and old slices is shown as a pie chart. The fraction of slices exhibiting ss LTP and r LTP were significantly different between the young and old preparations (χ2 = 5.45, two-tailed p = 0.0195 with Yates continuity correction) while those exhibiting no LTP were not (χ2 = 0.105, two-tailed p = 0.746 with Yates continuity correction). (c) Averages of the combined LTP responses (ss LTP and r LTP, excluding the no LTPs) show differences between the time course and magnitude of LTP between young and old slices. When ss LTP and r LTP were pooled together, the potentiation at 40 min after TBS was significantly larger in old slices (young: 1.67 ± 0.07 fold, n = 37 and old: 2.23 ± 0.16 fold, n = 44, p=0.0021, t=3.321, df=58.5, two-tailed unpaired t-test). Representative traces of fEPSPs are shown obtained at the indicated times “a”, just before the TBS, and “b”, at 40 min after the TBS. On the right panel, the box-and-whisker plots show the scatter of the fEPSP slopes relative to pre-TBS baseline, measured in individual experiments between 35-40 min after TBS. The dark symbols represent the experiments included in Fig.1a&b in which an untetanized pathway (S2) was also recorded. Note some very large LTP present in a few old slices that were not part of the two pathway experiments. The boxes of the plots delineate the 25-75% of the data, the lines in the middle are the medians, the crosses represent the means and the bars shown the 5-95% extent of the data spread.
Supplementary Figure 4 Paired-pulse facilitation (PPF) of the slopes of fEPSPs.
(a) Plots of the PPF ratios of the slope of the second fEPSP divided by the slope of the first fEPSP evoked by two pulses 50 ms apart. The fEPSPs were recorded before and 40 min after the TBS in young and old slices and are illustrated for the tetanized (S1) and untetanized (S2) pathways combined. LTP has a variable effect on PPF, as previously reported3, mainly depending on the value of PPF before the tetanic stimulation. A 2-way ANOVA of PPF ratios between young and old slices and the effects of TBS yielded no significant differences (effect of TBS F[1, 88]=0.9658, p=0.5284; effect of age F[1,88]=1.458, p=0.2306; and interaction TBS×age F[1,88]=2.009, p=0.1599). The PPF values for the S1 and S2 combined were: before TBS (bTBS), young 1.82 ± 0.07; old 1.85 ± 0.06; after TBS (aTBS), young 1.87 ± 0.16; old 1.63 ± 0.07). (b) We also compared in individual slices the PPF ratios before and after TBS. Overall, there were no significant effects of TBS on PPF in either preparation except in the PPF of S2 in the old slices (young, n=8: S1 bTBS 1.91 ± 0.13, 40 min aTBS 1.70 ± 0.24, p=0.5166, t=0.77, df=11; S2 bTBS 1.74 ± 0.09, aTBS 2.03 ± 0.22, p=0.2571, t=1.21, df=9.3; old, n=15: S1 bTBS 1.76 ± 0.04, aTBS 1.63 ± 0.10, p=0.243, t=1.21, df=18.4; S2 bTBS 1.93 ± 0.100, aTBS 1.64 ± 0.09, p=0.0227, t=2.417, df=27.7, all tests: paired two-tailed t-test).
Supplementary Figure 5 Effects of L-655,708 and d-AP5 on LTP in young and old slices.
(a) Plots of the averages of fEPSP slopes recorded before and after the TBS (indicated by ▲) in young slices in the presence of the GABAA receptor α5 subunit specific benzodiazepine inverse agonist L-655,708 (200 nM, “+L655”) and the competitive NMDA receptor antagonist d-AP5 (50 μM, “+d-AP5”). Control data from Supplementary Fig.3 are presented in a muted colors. L-655,708 had no significant effect on the potentiated fEPSP slope ratios at 40 min after TBS when compared to controls (1.78 ± 0.17, n=9 vs 1.67 ± 0.07, n = 37, p=0.563, t=0.207, df=10.9, unpaired t-test). As expected, LTP was blocked by d-AP5 as shown by the fEPSP slope ratios measured at 40 min after TBS (0.71 ± 0.22, n=6 vs 1.67 ± 0.07, n = 37, p=0.006, t=4.16, df=6.06, two-tailed unpaired t-test). Insets show superimposed typical fEPSPs recorded at the time points indicated by “a” and “b” for the two experimental conditions. Calibration bars: 0.5 mV, 5 ms. (b) Same as in A, for recordings in old slices. L655-708 significantly reduced the potentiated fEPSP slope ratios at 40 min after TBS when compared to controls (1.46 ± 0.14, n=11 vs 2.23 ± 0.16, n = 44, p=0.00086, t=3.622, df=38, two-tailed unpaired t-test), and as previously shown in old slices4, d-AP5 significantly reduced the magnitude of LTP, but did not fully block it (1.48 ± 0.14, n=9 vs 2.23 ± 0.16, n = 44, p=0.00148, t=3.478, df=32.3, two-tailed unpaired t-test). This residual LTP in old slices was significantly different from the d-AP5 blockade of LTP in the young (p=0.015, t=3.085, df=9, two-tailed unpaired t-test). Insets show superimposed typical fEPSPs recorded at the time points indicated by “a” and “b” for the two experimental conditions. Calibration bars: 0.5 mV, 5 ms.
Supplementary Figure 6 Effects of gabazine (GBZ) perfusion on fEPSPs before and after TBS evoked by stimulating the tetanized or the untetanized pathway in young and old slices.
(a) Plot of the effects of a pre-TBS gabazine (GBZ) perfusion on the fEPSPs slopes recorded in young but not old slices (in V/s, young: 0.59 ± 0.05 to 0.73 ± 0.06, n=17, p=0.0015, t=3.494, df=32, paired t-test; old: 0.43 ± 0.05 to 0.44 ± 0.06, n=14, p=0.65, t=0.459, df=26, paired t-test). The “before” condition refers to the average fEPSP slope measured over 5 min prior to GBZ perfusion, “after” refers to the slope averaged over 2 min starting at 4 min following GBZ perfusion. (b) Example experiments showing the effect of GBZ perfusion (grey bar) before TBS in young and old slices. The average of the slopes of the fEPSP recorded 10 min before TBS (indicated by ▲) are considered as 1.0 and are indicated by a dashed line. The lower dashed line denotes the average of the pre-GBZ baseline in the young slice. Note the absence of LTP in the old slice in the presence of GBZ. Insets show responses at the times marked with “a” and “b”. Calibration bars: 0.5 mV, 5 ms. (c) As in (a), but the plots show the effects of GBZ on fEPSP slopes 40 min after the LTP induced by TBS. GBZ perfusion did not affect fEPSP slopes after LTP in young slices (in V/s, 0.93 ± 0.12 to 0.90 ± 0.12, n=5, p=0.60, t=0.559, df=10, paired two-tailed t-test). In old slices, GBZ significantly reduced the fEPSP slopes in the tetanized pathway (in V/s, 1.01 ± 0.11 to 0.83 ± 0.15, n=14, p=0.0044, t=3.158, df=26, paired two-tailed t-test), but not in the potentiated untetanized pathway (in V/s, 0.83 ± 0.17 to 0.80 ± 0.22, n=6, p=0.63, t=0.499, df=10, paired two-tailed t-test). (d) Sample experiment showing the effect of GBZ perfusion (grey bar) on potentiated responses young slices. The average of the slopes of the fEPSP recorded 10 min before TBS (indicated by ▲) are considered as 1.0 and are indicated by a dashed line. Note the absence of LTP in the old slice in the presence of GBZ. Insets show responses at the times marked with “a” and “b”. Calibration bars: 0.5 mV, 5 ms, also apply to (e) and (f). (e) Representative experiment showing the effect of GBZ perfusion (grey bar) on the potentiated responses of the pathway receiving TBS (S1) in an old slice. Note the reduction of the fEPSP slope by GBZ. Insets show responses at the times marked with “a” and “b”. (f) Typical experiment showing the effect of GBZ perfusion (grey bar) on the potentiated responses of the pathway that did not receive TBS (S2) in an old slice. Note the lack of GBZ effect on the fEPSP. Insets show responses at the times marked with “a” and “b”.
Supplementary Figure 7 Properties of GABAergic events in young and old slices recorded in whole-cell configuration.
(a) Comparison of sIPSC frequencies shown similar values in young and old CA1 pyramidal cells (in s-1, young: 27.3 ± 6.3, n=6; old: 28.4 ± 4.2, n=15, p=0.969, Mann Whitney test). (b) Similarly, the sIPSC amplitudes were not different between the two preparations (in pA, young: 19.3 ± 1.8, n=6; old: 21 ± 1.3, n=15, p=0.5855, Mann Whitney test). (c) At the end of each experiment 100 μM picrotoxin was perfused onto the slices to reveal the tonic conductance in the cells. There were no differences in the tonic GABAA receptor-mediated currents when normalized to whole-cell capacitance (in A/F, young: 7.4 ± 1.5, n=6; old: 5.9 ± 0.6, n=15, p=0.4596, Mann Whitney test).
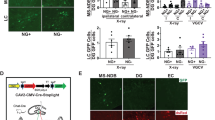
Supplementary Figure 8 Properties of LTP after loading CA1 pyramidal cells with Cl― by prolonged activation halorhodopsin (eNpHR3.0) in young slices.
(a) Expression of YFP and with it the linked eNpHR3.0 in the brains of F1 offspring of Camk2a-Cre/ERT2 mice (JAX Stock # 012362) crossed with Rosa-CAG-LSL-eNpHR3.0-EYFP-WPRE Ai39 mice (JAX Stock # 014539). Although the expression of eNpHR3.0 in these mice should be evident only after administration of tamoxifen, certain excitatory neuronal populations most notably in the hippocampal CA1 region, ventral thalamus, and some layers of the neocortex express YFP without the conditional inducer. (b) Higher magnification confocal image of the CA1 region showing expression in the somatic membrane and dendritic processes. (c) LTP induction in slices obtained from young eNpHR3.0 expressing mice following a 10-15 min continuous stimulation with 568 nm laser light to activate eNpHR3.0 and load the cells with Cl―. Measured 20 min after, the TBS induced a huge potentiation of both the tetanized (S1: 5.47±1.08 fold increase, n=4, significantly larger than in control young slices: p=0.043, t=3.384, df=3.25, two-tailed upaired t-test) and untetanized (S2: 4.90±1.32, n=4, not significantly different from S2 in old controls, p=0.0821, t=2.574, df=3.078, two-tailed upaired t-test) inputs, indicating that in Cl―-loaded cells the synapse specificity of LTP is lost. Notably, 3/4 experiments had to be terminated 25 min after TBS, as the slices exhibited spreading depression presumably due to excessive depolarization.
Supplementary Figure 9 Proposed mechanism for diminished KCC2 and GABAergic depolarization-induced spreading of LTP to unstimulated synapses.
During TBS in slices of young animals (top panels) the depolarization and Ca2+ spread are highly localized to the tetanized synapses (S1) confined to a small region of the dendritic tree. This is made possible by inhibitory GABAA receptors (blue) and functional dendritic KCC2 that prevents Cl― accumulation in the dendrites. Consequently, LTP can be considered a “clustered plasticity” (dashed box) that does not spread to the untetanized synapses (S2). In sharp contrast, in dendrites of old CA1 pyramidal cells and possibly due to reduced Ca2+ buffering, the Ca2+ signal induced by the TBS is larger and more widespread, leading to a reduced number or function of KCC2. This in turn renders the GABAA receptors depolarizing (red), spreading the dendritic depolarization even further to untetanized synapses (S2) resulting in their potentiation. The decreased KCC2 levels and depolarizing GABAA receptors appear to persist for at least 40 min at the site of the synapses receiving the TBS, but not at sites around the untetanized afferents. Thus the ensuing plasticity is confounded having become “unclustered”, i.e., it has spread to synapses that should have remained unrelated to the context of the original memory trace.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Tables 1–3. (PDF 2262 kb)
Rights and permissions
About this article
Cite this article
Ferando, I., Faas, G. & Mody, I. Diminished KCC2 confounds synapse specificity of LTP during senescence. Nat Neurosci 19, 1197–1200 (2016). https://doi.org/10.1038/nn.4357
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4357
This article is cited by
-
Intracellular chloride regulation mediates local sleep pressure in the cortex
Nature Neuroscience (2023)
-
Repurposing cancer drugs identifies kenpaullone which ameliorates pathologic pain in preclinical models via normalization of inhibitory neurotransmission
Nature Communications (2021)
-
Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization
Nature Communications (2020)
-
Perturbations in imprinted methylation from assisted reproductive technologies but not advanced maternal age in mouse preimplantation embryos
Clinical Epigenetics (2019)
-
Enhancing KCC2 function counteracts morphine-induced hyperalgesia
Scientific Reports (2017)