Abstract
Axonal damage has been associated with aberrant protein trafficking. We examined a newly characterized class of compounds that target nucleo-cytoplasmic shuttling by binding to the catalytic groove of the nuclear export protein XPO1 (also known as CRM1, chromosome region maintenance protein 1). Oral administration of reversible CRM1 inhibitors in preclinical murine models of demyelination significantly attenuated disease progression, even when started after the onset of paralysis. Clinical efficacy was associated with decreased proliferation of immune cells, characterized by nuclear accumulation of cell cycle inhibitors, and preservation of cytoskeletal integrity even in demyelinated axons. Neuroprotection was not limited to models of demyelination, but was also observed in another mouse model of axonal damage (that is, kainic acid injection) and detected in cultured neurons after knockdown of Xpo1, the gene encoding CRM1. A proteomic screen for target molecules revealed that CRM1 inhibitors in neurons prevented nuclear export of molecules associated with axonal damage while retaining transcription factors modulating neuroprotection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Trapp, B.D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Ferguson, B., Matyszak, M.K., Esiri, M.M. & Perry, V.H. Axonal damage in acute multiple sclerosis lesions. Brain 120, 393–399 (1997).
Nikic´, I. et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 17, 495–499 (2011).
Kim, J.Y. et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat. Neurosci. 13, 180–189 (2010).
Dormann, D. et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J. 29, 2841–2857 (2010).
Zhang, Z.C. & Chook, Y.M. Structural and energetic basis of ALS-causing mutations in the atypical proline-tyrosine nuclear localization signal of the Fused in Sarcoma protein (FUS). Proc. Natl. Acad. Sci. USA 109, 12017–12021 (2012).
Hutten, S. & Kehlenbach, R.H. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 17, 193–201 (2007).
Xu, D., Farmer, A. & Chook, Y.M. Recognition of nuclear targeting signals by Karyopherin-beta proteins. Curr. Opin. Struct. Biol. 20, 782–790 (2010).
Xu, D., Grishin, N.V. & Chook, Y.M. NESdb: a database of NES-containing CRM1 cargoes. Mol. Biol. Cell 23, 3673–3676 (2012).
Fung, H.Y. & Chook, Y.M. Atomic basis of CRM1-cargo recognition, release and inhibition. Semin. Cancer Biol. 27, 52–61 (2014).
Iqbal, K., Alonso Adel, C. & Grundke-Iqbal, I. Cytosolic abnormally hyperphosphorylated tau but not paired helical filaments sequester normal MAPs and inhibit microtubule assembly. J. Alzheimers Dis. 14, 365–370 (2008).
Alami, N.H. et al. Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron 81, 536–543 (2014).
Lagier-Tourenne, C., Polymenidou, M. & Cleveland, D.W. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 19, R46–R64 (2010).
Winton, M.J. et al. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J. Biol. Chem. 283, 13302–13309 (2008).
Kawai, Y., Garduno, L., Theodore, M., Yang, J. & Arinze, I.J. Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J. Biol. Chem. 286, 7629–7640 (2011).
Niture, S.K., Jain, A.K., Shelton, P.M. & Jaiswal, A.K. Src subfamily kinases regulate nuclear export and degradation of transcription factor Nrf2 to switch off Nrf2-mediated antioxidant activation of cytoprotective gene expression. J. Biol. Chem. 286, 28821–28832 (2011).
Linker, R.A. et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–692 (2011).
Jain, A.K., Bloom, D.A. & Jaiswal, A.K. Nuclear import and export signals in control of Nrf2. J. Biol. Chem. 280, 29158–29168 (2005).
Kudo, N. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96, 9112–9117 (1999).
Sun, Q. et al. Nuclear export inhibition through covalent conjugation and hydrolysis of Leptomycin B by CRM1. Proc. Natl. Acad. Sci. USA 110, 1303–1308 (2013).
Lapalombella, R. et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 120, 4621–4634 (2012).
Etchin, J. et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 27, 66–74 (2013).
Recks, M.S. et al. Early axonal damage and progressive myelin pathology define the kinetics of CNS histopathology in a mouse model of multiple sclerosis. Clin. Immunol. 149, 32–45 (2013).
Sakakibara, K. et al. CBS9106 is a novel reversible oral CRM1 inhibitor with CRM1 degrading activity. Blood 118, 3922–3931 (2011).
Bloss, E.B. & Hunter, R.G. Hippocampal kainate receptors. Vitam. Horm. 82, 167–184 (2010).
Basso, D.M. et al. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 (2006).
Merkler, D., Ernsting, T., Kerschensteiner, M., Bruck, W. & Stadelmann, C. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain 129, 1972–1983 (2006).
Chaudhary, P. et al. Lipoic Acid reduces inflammation in a mouse focal cortical experimental autoimmune encephalomyelitis model. Neurology 82, P1.221 (2014).
Li, A. et al. Upregulation of CRM1 relates to neuronal apoptosis after traumatic brain injury in adult rats. J. Mol. Neurosci. 51, 208–218 (2013).
Mastroeni, D. et al. Reduced RAN expression and disrupted transport between cytoplasm and nucleus; a key event in Alzheimer's disease pathophysiology. PLoS ONE 8, e53349 (2013).
Nagara, Y. et al. Impaired cytoplasmic-nuclear transport of hypoxia-inducible factor-1alpha in amyotrophic lateral sclerosis. Brain Pathol. 23, 534–546 (2013).
Dormann, D. & Haass, C. TDP-43 and FUS: a nuclear affair. Trends Neurosci. 34, 339–348 (2011).
Kim, J.Y. & Casaccia, P. HDAC1 in axonal degeneration: a matter of subcellular localization. Cell Cycle 9, 3680–3684 (2010).
Zhang, J. & Herrup, K. Nucleocytoplasmic Cdk5 is involved in neuronal cell cycle and death in post-mitotic neurons. Cell Cycle 10, 1208–1214 (2011).
Sultan, A. et al. Nuclear tau, a key player in neuronal DNA protection. J. Biol. Chem. 286, 4566–4575 (2011).
Ke, Y.D. et al. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer's and Pick's disease. PLoS ONE 7, e35678 (2012).
Aggarwal, A. & Agrawal, D.K. Importins and exportins regulating allergic immune responses. Mediators Inflamm. 2014, 476357 (2014).
Pemberton, L.F. & Paschal, B.M. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6, 187–198 (2005).
Shriver, L.P. & Dittel, B.N. T-cell-mediated disruption of the neuronal microtubule network: correlation with early reversible axonal dysfunction in acute experimental autoimmune encephalomyelitis. Am. J. Pathol. 169, 999–1011 (2006).
Goldmann, T. & Prinz, M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013, 208093 (2013).
Campbell, G.R. & Mahad, D.J. Mitochondria as crucial players in demyelinated axons: lessons from neuropathology and experimental demyelination. Autoimmune Dis. 2011, 262847 (2011).
Stankiewicz, J.M., Kolb, H., Karni, A. & Weiner, H.L. Role of immunosuppressive therapy for the treatment of multiple sclerosis. Neurotherapeutics 10, 77–88 (2013).
Tedeholm, H. et al. Time to secondary progression in patients with multiple sclerosis who were treated with first generation immunomodulating drugs. Mult. Scler. 19, 765–774 (2013).
Rawji, K.S. & Yong, V.W. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin. Dev. Immunol. 2013, 948976 (2013).
Koyama, M. & Matsuura, Y. An allosteric mechanism to displace nuclear export cargo from CRM1 and RanGTP by RanBP1. EMBO J. 29, 2002–2013 (2010).
Vaguine, A.A., Richelle, J. & Wodak, S.J. SFCHECK: a unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr. D Biol. Crystallogr. 55, 191–205 (1999).
Bernard, C.C. et al. Myelin oligodendrocyte glycoprotein: a novel candidate autoantigen in multiple sclerosis. J. Mol. Med. 75, 77–88 (1997).
Stoppini, L., Buchs, P.A. & Muller, D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37, 173–182 (1991).
Vidaurre, O.G. et al. Cerebrospinal fluid ceramides from patients with multiple sclerosis impair neuronal bioenergetics. Brain 137, 2271–2286 (2014).
la Cour, T. et al. Analysis and prediction of leucine-rich nuclear export signals. Protein Eng. Des. Sel. 17, 527–536 (2004).
Acknowledgements
We thank T. Flagiello and X. Pedre for assistance with EAE animal experiments. The project was supported by US National Institutes of Health grants R01-NS69385 and R37-NS42925, seed funds from Karyopharm Therapeutics to P.C., and the Fast Forward division of the National Multiple Sclerosis Society to P.C. and S.S. T.K. was supported by funds from the Interdisciplinary Centre for Clinical Research in Münster (KuT3/006/11). O.H. is the recipient of the Mount Sinai Helmsley Award. J.D.H. holds a postdoctoral fellowship from the Multiple Sclerosis Society of Canada and the Fonds de la recherche en santé du Québec. Human tissue samples for western blotting were supplied by the UK Multiple Sclerosis Tissue Bank, funded by the MS Society of Great Britain and Northern Ireland, registered charity 207495.
Author information
Authors and Affiliations
Contributions
P.C., S.S., J.D.H. and D.M. were responsible for overall analysis and study design. In vivo and in vitro experiments were performed by J.D.H., O.H., B.d.l.H., O.G.V. and G.A.M. Q.S., H.Y.J.F. and Y.M.C. crystallized CRM1 bound to SINE and performed gel shift assays. S.A. and T.K. performed the human brain immunohistochemistry experiments. G.J.K. helped analyze and interpret the three-dimensional electron microscopy results. Immunology experiments were conceived and interpreted by O.H. and K.A. Pharmacokinetic experiments and characterization of drug properties were performed by D.M. and S.S. The paper was written by J.D.H. and P.C.
Corresponding author
Ethics declarations
Competing interests
S.S. and D.M. are currently employed at and hold leadership positions at Karyopharm Therapeutics, as well as hold stock in the company.
Integrated supplementary information
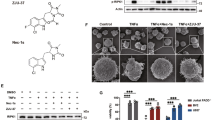
Supplementary Figure 1 Comparison of KPT-276, KPT-185 and KPT-251 bound to CRM1.
(a-c) Chemical structure of the KPT-276 compared to the structure of related compounds KPT-185 and KPT-251 inhibitors, (d-f). Views of the NES-binding groove of CRM1, showing interactions between CRM1 and three different KPT inhibitors. Interactions (≤4.0 Å) between KPT-276 (d, magenta), KPT-185 (e, orange) and KPT-251 (f, gray) and CRM1 (pink) are shown as gray dashed lines. (g-i) Views of the complexes generated by 90° rotations about the vertical axes relative to the views in (d-f). Helices H12A in the CRM1 grooves were removed for clarity. The variable arm of KPT-276 binds in a different orientation from those of the previously reported KPT-185 and KPT-251.
Supplementary Figure 2 CRM1 is ubiquitously expressed in cells of the central nervous system and periphery, and KPT inhibitors are not toxic to in vitro cultured cells.
(a,b) Xpo1 (CRM1) mRNA levels in CNS neurons and glia (oligodendrocytes, microglia, astrocytes) and peripheral immune cells (total splenocytes, CD19+ B cells, CD4+ T cells, CD8+ T cells, CD11b+ monocytes/microglia, and CD11c+ dendritic cells) all normalized to Gapdh. MTT assay of (c) spinal cord neurons, (d) cortical neurons, (e) oligodendrocyte progenitors, (f) oligodendrocytes, (g) astrocytes, or (h) splenocytes treated with the indicated doses of KPT-276 and KPT-350 (concentration range 0.1 to 1000 nM) for 24 hr. Bar graphs represent mean ± SEM, n = 8 samples per conditions, from two independent experiments. Statistical differences in all graphs were determined using one-way ANOVA with Dunnett’s correction (***p < 0.001).
Supplementary Figure 3 Therapeutic treatment of EAE mice with KPT compounds does not negatively impact body weight or body condition.
Mice treated after the development of hindlimb paralysis were also monitored for (a) body weight and (b) body condition. Arrows indicate the start and end of the treatment. Mice were supplemented with softened food and a high calorie nutritional supplement (NutricalR) in order to maintain healthy body weight. Body condition 3 = normal; 1 = anorexic; 5 = obese. (c) FluoroMyelin stained longitudinal sections of EAE spinal cords; scale bear = 200 µm. (d) Quantification of FluoroMyelin intensity from EAE animals. The bar graphs represent mean ± SEM. Statistical differences in: (a), (b) were determined by performing linear regression analysis on the data points during the drug treatment period window; (d) were determined using one-way ANOVA with Dunnett’s correction (**p < 0.01, ***p < 0.001 vs. vehicle).
Supplementary Figure 4 KPT inhibitors do not affect transcript levels of oligodendrocyte lineage markers, nor differentiation or oligodendrocyte progenitor proliferation.
(a) Equal weights of spinal cord tissue was used to determine relative transcript levels of stage specific markers of the oligodendrocyte lineage, in EAE mice treated therapeutically with either vehicle, or KPT-350, normalized to 18S rRNA, n=8 per condition, from two independent experiments. (b) Representative images of oligodendrocytes treated with KPT-276 (10 nM) or KPT-350 (10 nM). Cells were grown in the presence of the KPT for 48 hr and the differentiation markers O4 (green) and MBP (red) were determined. Cells were counterstained with DAPI (blue); scale bar represents 50 μm. (c) Quantification of cells in (b). (d) Values represent mean ± SEM of three fields, from three independent experiments. Statistical differences in (a) were determined using independent t-tests with Bonferroni correction; (c), (d) one-way ANOVA with Dunnett’s correction.
Supplementary Figure 5 Therapeutic treatment of EAE mice with KPT inhibitors reduces total splenocytes and maintains relatively equal proportions of splenic immune cell populations.
(a) Representative images of spleens from vehicle, KPT-276 and KPT-350-treated animals, harvested at d28 of EAE, scale bar = 0.5 cm. (b) Quantification of total splenocytes from spleens of vehicle, KPT-276 and KPT-350-treated animals. (c) Pie graphs depicting the relative proportions of splenic immune cell populations in vehicle, KPT-276, or KPT-350-treated animals. Bar graphs represent mean ± SEM. Statistical differences in: (b) were determined using one-way ANOVA with Dunnett’s correction (***p < 0.001).
Supplementary Figure 6 Prophylactic treatment with KPT-276 and KPT-350 at timing of immunization attenuates disease onset and immune cell numbers in the spinal cord and periphery.
(a) Schematic diagram of treatment paradigm for prophylactic EAE. Clinical scores from prophylactic treatment of 8-week old C57/BL6 female mice with MOG35-55-induced EAE and gavaged either with vehicle, KPT-276, or KPT-350 starting at immunization; drug-treatment time window is indicated by the arrow. The graph represents the average mean value of the clinical score ± SEM, n=12 mice per group. (b) Cumulative clinical scores in mice treated from the time of immunization, n=8 animals per group. (c) Mouse body weight and (d) body condition. Arrows indicate the start and end of the treatment. Mice were supplemented with softened food and a high calorie nutritional supplement (NutricalR) in order to maintain healthy body weight. Body condition 3 = normal; 1 = anorexic; 5 = obese. (e) Quantification by flow cytometry of immune cell infiltrates in spinal cords of mice. Microglial cells were identified as CD11b+/ CD45low and monocytes as CD11b+/CD45high (n=4 mice per condition). (f) Quantification of total splenocytes. (g) Quantification by flow cytometry of splenic populations in vehicle, KPT-276 or KPT-350 treated mice. Monocytes were identified as CD11b+/ CD115+, neutrophils as CD11b+/CD115- and dendritic cells (DC) as CD11c+/MHCIIhigh. All bar graphs represent mean ± SEM. Statistical differences in: (b), (e), (f), (g) were determined using Kruskal-Wallis test with Dunn’s correction (*p < 0.05, **p < 0.01, ***p < 0.001 all relative to vehicle); (c), (d) were determined by performing linear regression analysis on the data points during the drug treatment period window.
Supplementary Figure 7 Washout of KPT inhibitors in naive mice restores spleen immune cell populations.
(a) Schematic diagram of method to assess toxicity of drug in spleen. Quantification of total splenocytes in KPT-276, KPT-276 washout, KPT-350, or KPT-350 washout. (b) Quantification by flow cytometry of splenic populations in vehicle, KPT-276 or KPT-350 treated mice. Animals were treated for 14d with drug, or an identical cohort of animals treated with the compounds followed by drug washout for 14d. Monocytes were identified as CD11b+/ CD115+, neutrophils as CD11b+/CD115- and dendritic cells (DC) as CD11c+/MHCIIhigh. Bar graphs represent mean ± SEM. Statistical differences in: (a) were determined using Kruskal-Wallis test with Dunn’s correction (*p < 0.05 vs. vehicle); (b) were determined using independent t-tests with Bonferroni correction (*p < 0.05, **p < 0.01, ***p < 0.001 all relative to the non-washout cell population).
Supplementary Figure 8 Neurofilament heavy chain transcript levels are upregulated in EAE mice treated with KPT-350.
(a) Equal weight of spinal cord tissue was used to determine relative transcript levels of stage specific markers of the oligodendrocyte lineage, and of neurofilament heavy chain (Nefh) in mice treated either with vehicle, or KPT-350, normalized to 18S rRNA, n=8 per condition, from two independent experiments. The bar graph represents mean ± SEM. Statistical difference was determined using an independent t-test (***p < 0.001 vs vehicle).
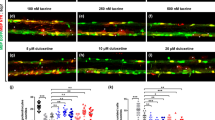
Supplementary Figure 9 KPT-350 treatment of mouse hippocampal slice cultures prevents the induction of focal axonal damage in models independent of inflammation.
(a) Schematic diagram of kainic-acid model of induced neurotoxicity in mouse hippocampal slice cultures. Representative confocal images of the hippocampal CA3 region from cultures treated with KPT-350 (10 nM) for 1 hr, followed by exposure to 5 µM kainic acid for 18 hr. Slices were stained with NFH (red) to label axons, SMI-32 (green) to label damaged axons, DAPI (blue) was used to counterstain nuclei; scale bar = 100 µm. (b) Quantification of SMI-32 intensity in (a). Values represent mean pixel intensity ± SEM of n= 3 slices per group from three independent experiments. Statistical differences in (b) were determined using one-way ANOVA with Dunnett’s correction (***p < 0.001 vs. kainic acid).
Supplementary Figure 10 KPT-350 prevents the decrease in mitochondrial spare respiratory capacity induced by excitotoxic and inflammatory damage.
(a) Mitochondrial respiratory function was determined by measuring the oxygen consumption rate of neurons treated with DMSO, glut+TNFα in the absence or presence of KPT (10nM of KPT-350) using a SeaHorse Bioanalyzer. (b) Quantification of spare respiratory capacity of neurons treated in (a). Statistical differences in (b) were determined using one-way ANOVA with Dunnett’s correction (*p < 0.05 vs. DMSO).
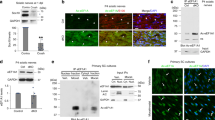
Supplementary Figure 11 Full length western blots for human data showing increased expression of CRM1 in MS gray matter.
Full length western blots for human data (corresponding to Figure 1e).
Supplementary Figure 12 Full length western blots from rat neurons to validate CRM1 cargos
Full length western blots for human data (corresponding to Figure 8b).
Supplementary Figure 13 Full length western blots from experiments on EAE mice to validate of CRM1 cargoes
Full length western blots for human data (corresponding to Figure 8d).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1–3 (PDF 2705 kb)
Video of EAE mice taken at drug start time, on day 16 post-immunization with MOG35-55 when mice exhibited hindlimb paralysis.
Mice were started on treatment at d16 post-immunization, and were oral gavaged with vehicle three times per week until d28. (MOV 3089 kb)
Video of therapeutic EAE mice treated with vehicle.
Video was taken at d28 post-immunization with MOG35-55. Mice were started on treatment at d16 post-immunization, and were oral gavaged with vehicle three times per week until d28. (MP4 1889 kb)
Video of therapeutic EAE mice treated with KPT-276 (75 mg/kg).
Video was taken at d28 post-immunization with MOG35-55. Mice were started on treatment at d16 post-immunization, and were oral gavaged with KPT-276 three times per week until d28. (MP4 5856 kb)
Video of therapeutic EAE mice treated with KPT-350 (7.5 mg/kg).
Mice were started on treatment at d16 post-immunization, and were oral gavaged with KPT-350 three times per week until d28. (MP4 7075 kb)
Rights and permissions
About this article
Cite this article
Haines, J., Herbin, O., de la Hera, B. et al. Nuclear export inhibitors avert progression in preclinical models of inflammatory demyelination. Nat Neurosci 18, 511–520 (2015). https://doi.org/10.1038/nn.3953
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3953
This article is cited by
-
Trait − driven analysis of the 2p15p16.1 microdeletion syndrome suggests a complex pattern of interactions between candidate genes
Genes & Genomics (2023)
-
Substance P Reduces Infarct Size and Mortality After Ischemic Stroke, Possibly Through the M2 Polarization of Microglia/Macrophages and Neuroprotection in the Ischemic Rat Brain
Cellular and Molecular Neurobiology (2023)
-
The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury
Nature Communications (2022)
-
Karyopherin-mediated nucleocytoplasmic transport
Nature Reviews Molecular Cell Biology (2022)
-
Triad of TDP43 control in neurodegeneration: autoregulation, localization and aggregation
Nature Reviews Neuroscience (2021)