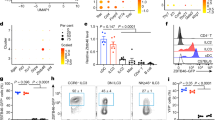
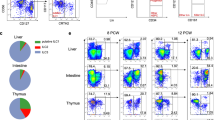
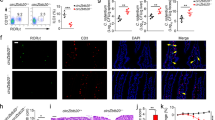
Abstract
Innate lymphoid cells (ILCs) communicate with other hematopoietic and nonhematopoietic cells to regulate immunity, inflammation and tissue homeostasis. How ILC lineages develop and are maintained remains largely unknown. In this study we observed that a divergent long noncoding RNA (lncRNA), lncKdm2b, was expressed at high levels in intestinal group 3 ILCs (ILC3s). LncKdm2b deficiency in the hematopoietic system led to reductions in the number and effector functions of ILC3s. LncKdm2b expression sustained the maintenance of ILC3s by promoting their proliferation through activation of the transcription factor Zfp292. Mechanistically, lncKdm2b recruited the chromatin organizer Satb1 and the nuclear remodeling factor (NURF) complex onto the Zfp292 promoter to initiate its transcription. Deletion of Zfp292 or Bptf also abrogated the maintenance of ILC3s, leading to susceptibility to bacterial infection. Therefore, our findings reveal that lncRNAs may represent an additional layer of regulation of ILC development and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Buonocore, S. et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 (2010).
Ebihara, T. et al. Runx3 specifies lineage commitment of innate lymphoid cells. Nat. Immunol. 16, 1124–1133 (2015).
Bernink, J.H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
Brestoff, J.R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015).
Yagi, R. et al. The transcription factor GATA3 is critical for the development of all IL-7Rα-expressing innate lymphoid cells. Immunity 40, 378–388 (2014).
Eberl, G. et al. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Guo, X. et al. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity 42, 731–743 (2015).
Guo, X. et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39 (2014).
Song, C. et al. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J. Exp. Med. 212, 1869–1882 (2015).
Diefenbach, A., Colonna, M. & Koyasu, S. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41, 354–365 (2014).
Serafini, N. et al. Gata3 drives development of RORγt+ group 3 innate lymphoid cells. J. Exp. Med. 211, 199–208 (2014).
De Obaldia, M.E. & Bhandoola, A. Transcriptional regulation of innate and adaptive lymphocyte lineages. Annu. Rev. Immunol. 33, 607–642 (2015).
Klose, C.S. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Constantinides, M.G., McDonald, B.D., Verhoef, P.A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397–401 (2014).
Sanos, S.L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Kiss, E.A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Zhong, C. et al. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat. Immunol. 17, 169–178 (2016).
Batista, P.J. & Chang, H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell 152, 1298–1307 (2013).
Djebali, S. et al. Landscape of transcription in human cells. Nature 489, 101–108 (2012).
Kretz, M. et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 493, 231–235 (2013).
Zhu, P. et al. lnc-β-Catm elicits EZH2-dependent β-catenin stabilization and sustains liver CSC self-renewal. Nat. Struct. Mol. Biol. 23, 631–639 (2016).
Zhu, P. et al. LncBRM initiates YAP1 signalling activation to drive self-renewal of liver cancer stem cells. Nat. Commun. 7, 13608 (2016).
Luo, S. et al. Divergent lncRNAs regulate gene expression and lineage differentiation in pluripotent cells. Cell Stem Cell 18, 637–652 (2016).
Anderson, K.M. et al. Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436 (2016).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Klose, C.S. et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Katayama, S. et al. Antisense transcription in the mammalian transcriptome. Science 309, 1564–1566 (2005).
Yasui, D., Miyano, M., Cai, S., Varga-Weisz, P. & Kohwi-Shigematsu, T. SATB1 targets chromatin remodelling to regulate genes over long distances. Nature 419, 641–645 (2002).
McKenzie, A.N., Spits, H. & Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 41, 366–374 (2014).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
De Togni, P. et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science 264, 703–707 (1994).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725 (2009).
Sawa, S. et al. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330, 665–669 (2010).
Sonnenberg, G.F., Fouser, L.A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 (2011).
Guttman, M. et al. Ab initio reconstruction of cell type–specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 28, 503–510 (2010).
Cai, S., Han, H.J. & Kohwi-Shigematsu, T. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat. Genet. 34, 42–51 (2003).
Savarese, F. et al. Satb1 and Satb2 regulate embryonic stem cell differentiation and Nanog expression. Genes Dev. 23, 2625–2638 (2009).
Beyer, M. et al. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat. Immunol. 12, 898–907 (2011).
Will, B. et al. Satb1 regulates the self-renewal of hematopoietic stem cells by promoting quiescence and repressing differentiation commitment. Nat. Immunol. 14, 437–445 (2013).
Satoh, Y. et al. The Satb1 protein directs hematopoietic stem cell differentiation toward lymphoid lineages. Immunity 38, 1105–1115 (2013).
Steidl, U. et al. A distal single nucleotide polymorphism alters long-range regulation of the PU.1 gene in acute myeloid leukemia. J. Clin. Invest. 117, 2611–2620 (2007).
Hartley, P.D. & Madhani, H.D. Mechanisms that specify promoter nucleosome location and identity. Cell 137, 445–458 (2009).
Clapier, C.R. & Cairns, B.R. Regulation of ISWI involves inhibitory modules antagonized by nucleosomal epitopes. Nature 492, 280–284 (2012).
Tosi, A. et al. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell 154, 1207–1219 (2013).
Ruthenburg, A.J. et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell 145, 692–706 (2011).
Hamiche, A., Sandaltzopoulos, R., Gdula, D.A. & Wu, C. ATP-dependent histone octamer sliding mediated by the chromatin remodeling complex NURF. Cell 97, 833–842 (1999).
Landry, J. et al. Essential role of chromatin remodeling protein Bptf in early mouse embryos and embryonic stem cells. PLoS Genet. 4, e1000241 (2008).
Song, H., Spichiger-Haeusermann, C. & Basler, K. The ISWI-containing NURF complex regulates the output of the canonical Wingless pathway. EMBO Rep. 10, 1140–1146 (2009).
Zhu, X. et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci. Rep. 4, 6420 (2014).
Lee, J.S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–151 (2011).
Ye, B. et al. Cytosolic carboxypeptidase CCP6 is required for megakaryopoiesis by modulating Mad2 polyglutamylation. J. Exp. Med. 211, 2439–2454 (2014).
Chu, C., Qu, K., Zhong, F.L., Artandi, S.E. & Chang, H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 44, 667–678 (2011).
Acknowledgements
We thank D.R. Littman (New York University, New York, New York, USA) for providing pMIGR plasmid, and P. Zhu (Institute of Biophysics, CAS, Beijing, China) for pcDNA4/TO-mSatb1 plasmid. C. rodentium was a gift from B. Ge (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China). We thank J. Li (Cnkingbio Company Ltd., Beijing, China) for technical support. We also thank S. Meng, D. Fan, X. Wu, L. Zhou, Y. Teng and J. Jia for technical support. This work was supported by the National Natural Science Foundation of China (grants 91640203, 31530093, and 91419308 to Z.F.; 31470864 and 31670886 to B.Y.; and 31601189 to X.Z.) and the Strategic Priority Research Programs of the Chinese Academy of Sciences (grants XDB19030203 and XDA12020219 to Z.F.).
Author information
Authors and Affiliations
Contributions
B.L. designed and conducted experiments, analyzed data and wrote the paper; B.Y. carried out experiments and analyzed data; L.Y. and X.Z. constructed plasmids and established mouse tools; G.H., P. Z., J.W., Y.D. and X.Q. analyzed data; R.C. initiated the study and analyzed data; Y.T. designed animal experiments and analyzed data; and Z.F. initiated the study and organized, designed, and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Construction of lncKdm2b–RFP reporter and lncKdm2b-knockout mice.
(a) ILC3s (Lin−CD45loCD90hi) were isolated from small intestines, followed by immunofluorescence staining. LncKdm2b was detected by RNA fluorescence in situ hybridization (RNA-FISH). Green: lncKdm2b probe or scramble probe; red: CD127 or rat IgG2a-κ; purple: CD45 or rat IgG2b-κ. Nuclei were counterstained by DAPI. Scale bar, 5 μm. (b) Strategies for construction of lncKdm2bflox/flox and lncKdm2bRFP mice. (c) Total RNA from BM cells of lncKdm2bflox/flox or lncKdm2bflox/flox;Vav-Cre mice were extracted, followed by RNA hybridization. LncKdm2b specific probe was labeled with biotin. 18S RNA served as a loading control. (d,e) Analysis of ILC1s (d) and ILC2s (e) in small intestines from lncKdm2bflox/flox or lncKdm2bflox/flox;Vav-Cre mice by FACS. ILC1s were gated on CD3ɛ−CD45+NK1.1+NKp46+ and ILC2s were gated on Lin−Thy1.2+CD25+KLRG1+ST2+. n=6 for each group. (f,g) Analysis of NK cells in spleen (f) and ILC1s in liver (g) from lncKdm2bflox/flox;Vav-Cre− or lncKdm2bflox/flox;Vav-Cre+ mice. NK cells were gated on NKp46+NK1.1+. ILC1s were gated on CD3ɛ−DX5−TCRβ−NK1.1+CD49a+. n=6 for each group. (h) Total RNAs were extracted from ILC3s derived from lncKdm2bflox/flox or lncKdm2bflox/floxRorc-Cre mice, followed by analysis of lncKdm2b expression with RT-qPCR. (i) Numbers of NKp46+ ILC3s, CD4+ ILC3s and CD4− ILC3s in lncKdm2b+/+ and lncKdm2b-/- mice were calculated and shown as means±SD. n=6 per group. (j,k) LncKdm2bflox/flox and lncKdm2bflox/floxRorc-Cre mice were infected with C. rodentium. 6 days later, bacterial counts (CFUs) in feces (j) and colon length (k) were measured. n=6 for each group. (l) LPLs were isolated from lncKdm2b+/+ and lncKdm2b-/- mice post C. rodentium infection, and stimulated with IL-23 for 4 h. Then IL-22+ ILC3s were analyzed by FACS. (m) Secreted IL-22 protein by LPLs as in l was tested by ELISA after stimulated with IL-23 for 24h. n=6 each group. *p<0.01 and **p<0.001 by two-tailed Student’s t test. All data are representative of three independent experiments.
Supplementary Figure 2 LncKdm2b interacts with Sabt1.
(a) BrdU+ ILC3s (Lin−CD45+CD127+RORγt+) were analyzed by FACS. n=6 for each group. (b) Percentages of total ILC3s (Lin−CD45.2+CD127+RORγt+) in chimeras were checked by FACS. n=6 for each group. (c) Expression levels of lncKdm2b neighboring genes in lncKdm2b+/+ and lncKdm2b−/-ILC3s were detected by RT-qPCR. Data were normalized to endogenous Actb and shown as means ± SD. (d) Identification of Satb1 as an lncKdm2b binding protein. Satb1 protein sequences were identified by LTQ Orbitrap XL. (e) Relative mRNA levels of Zfp292 were examined by real-time qPCR. Fold changes were normalized to endogenous Actb. (f) Enrichment of RNA pol II on Zfp292 promoter in lncKdm2b+/+ and lncKdm2b-/- ILC3s. Nuclei were extracted from lncKdm2b+/+ or lncKdm2b-/-ILC3s, followed by ChIP-qPCR assays. (g) Generation of Satb1-/- mice. The schematic diagram of Satb1 KO construction by CRISPR/Cas9 technology (upper left). Frameshift of Satb1 gene was confirmed by DNA sequencing (lower left). Satb1 deletion was validated by immunoblotting (right panel). *p<0.01 by two-tailed Student’s t test. All data are representative of three independent experiments.
Supplementary Figure 3 Sabt1 interacts with the NURF complex.
(a) Identification of Bptf and Snf2l as Satb1 binding proteins. Bptf and Snf2l protein sequences were identified by LTQ Orbitrap XL. (b) Generation of Zfp292-/- mice. The schematic diagram of Satb1 KO construction by CRISPR/Cas9 technology (upper left). Frameshift of Zfp292 gene was confirmed by DNA sequencing (lower left). Zfp292 deletion was verified by immunoblotting (right panel). (c) LPLs from Zfp292+/+ and Zfp292-/- mice were isolated and stimulated with IL-23 for 4 h. Numbers of IL-22+ ILC3s were examined by FACS. *p<0.01 by two-tailed Student’s t test. All data are representative of three independent experiments.
Supplementary Figure 4 Deletion of Sabt1 or Bptf leads to reduced numbers of ILC3s.
(a,b) Percentages (a) and numbers (b) of ILC3s in small intestines derived from Satb1+/+ and Satb1-/- mice. Percentages of indicated cells were calculated and shown at right panel (a). n=6 per group. (c,d) Percentages (c) and numbers (d) of ILC3s in small intestines derived from Bptfflox/flox and Bptfflox/floxRorc-Cre mice. Percentages of indicated cells were calculated and shown at right panel (c). n=6 per group. (e) Bptfflox/flox and Bptfflox/floxRorc-Cre mice were infected with C. rodentium (5x109 CFUs per mouse). 6 days later, bacterial counts (CFUs) in feces were measured. n=6 for each group. (f) Bptfflox/floxRorc-Cre− and Bptfflox/floxRorc-Cre+ mice were infection with C. rodentium (5x109 CFUs per mouse) for 6 days, followed by H&E staining for colons. Scale bar, 50 μm. (g) 1x106 CD45.1 BM cells with 1x106 CD45.2 LPLs from WT or TKO mice infected with Zfp292 expressing retroviral were transplanted into lethally irradiated CD45.1 recipients. Percentages of ILC3s derived from CD45.2+ donors were analyzed by FACS. n=6 for each group. (h) Numbers of ILC3s as in g were analyzed by FACS. n=6 for each group. (i) Percentages of Ki67+ ILC3s in Zfp292+/+, lncKdm2b overexpression in Zfp292+/+ or Zfp292-/- LPLs were examined by FACS. n=6 for each group. *p<0.01 and **p<0.001 by two-tailed Student’s t test. All data are representative of at least three independent experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1 and 2 (PDF 1037 kb)
Rights and permissions
About this article
Cite this article
Liu, B., Ye, B., Yang, L. et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol 18, 499–508 (2017). https://doi.org/10.1038/ni.3712
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3712
This article is cited by
-
M6A-mediated-upregulation of lncRNA BLACAT3 promotes bladder cancer angiogenesis and hematogenous metastasis through YBX3 nuclear shuttling and enhancing NCF2 transcription
Oncogene (2023)
-
Regulation of noncoding RNAs in innate lymphoid cells
Cellular & Molecular Immunology (2023)
-
HOXA-AS2 contributes to regulatory T cell proliferation and immune tolerance in glioma through the miR-302a/KDM2A/JAG1 axis
Cell Death & Disease (2022)
-
WTAP-mediated m6A modification of lncRNA DIAPH1-AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis
Cell Death & Differentiation (2022)
-
Circular RNA circTmem241 drives group III innate lymphoid cell differentiation via initiation of Elk3 transcription
Nature Communications (2022)