Abstract
The mouse thymus produces discrete γδ T cell subsets that make either interferon-γ (IFN-γ) or interleukin 17 (IL-17), but the role of the T cell antigen receptor (TCR) in this developmental process remains controversial. Here we show that Cd3g+/− Cd3d+/− (CD3 double-haploinsufficient (CD3DH)) mice have reduced TCR expression and signaling strength on γδ T cells. CD3DH mice had normal numbers and phenotypes of αβ thymocyte subsets, but impaired differentiation of fetal Vγ6+ (but not Vγ4+) IL-17-producing γδ T cells and a marked depletion of IFN-γ-producing CD122+ NK1.1+ γδ T cells throughout ontogeny. Adult CD3DH mice showed reduced peripheral IFN-γ+ γδ T cells and were resistant to experimental cerebral malaria. Thus, TCR signal strength within specific thymic developmental windows is a major determinant of the generation of proinflammatory γδ T cell subsets and their impact on pathophysiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Wang, T. et al. IFN-γ-producing γδ T cells help control murine West Nile virus infection. J. Immunol. 171, 2524–2531 (2003).
Gao, Y. et al. γδ T cells provide an early source of interferon-γ in tumor immunity. J. Exp. Med. 198, 433–442 (2003).
Jagannathan, P. et al. Loss and dysfunction of Vδ2+ γδ T cells are associated with clinical tolerance to malaria. Sci. Transl. Med. 6, 251ra117 (2014).
Cho, J.S. et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Invest. 120, 1762–1773 (2010).
Sheridan, B.S. et al. γδ T cells exhibit multifunctional and protective memory in intestinal tissues. Immunity 39, 184–195 (2013).
Cai, Y. et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 35, 596–610 (2011).
Pantelyushin, S. et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 122, 2252–2256 (2012).
Cai, Y. et al. Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat. Commun. 5, 3986 (2014).
Park, S.G. et al. T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity 33, 791–803 (2010).
Romani, L. et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451, 211–215 (2008).
Shichita, T. et al. Pivotal role of cerebral interleukin-17-producing γδ T cells in the delayed phase of ischemic brain injury. Nat. Med. 15, 946–950 (2009).
Jensen, K.D. et al. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon-γ. Immunity 29, 90–100 (2008).
Sutton, C.E. et al. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341 (2009).
Petermann, F. et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity 33, 351–363 (2010).
Prinz, I., Silva-Santos, B. & Pennington, D.J. Functional development of γδ T cells. Eur. J. Immunol. 43, 1988–1994 (2013).
Ribot, J.C. et al. CD27 is a thymic determinant of the balance between interferon-γ– and interleukin 17-producing γδ T cell subsets. Nat. Immunol. 10, 427–436 (2009).
Haas, J.D. et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing γδ effector T cells. Eur. J. Immunol. 39, 3488–3497 (2009).
Rei, M. et al. Murine CD27− Vγ6+ γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc. Natl. Acad. Sci. USA 111, E3562–E3570 (2014).
Wu, P. et al. γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40, 785–800 (2014).
Silva-Santos, B., Serre, K. & Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 15, 683–691 (2015).
Turchinovich, G. & Hayday, A.C. Skint-1 identifies a common molecular mechanism for the development of interferon-γ–secreting versus interleukin-17-secreting γδ T cells. Immunity 35, 59–68 (2011).
Wencker, M. et al. Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol. 15, 80–87 (2014).
Ciofani, M. & Zúñiga-Pflücker, J.C. Determining γδ versus αβ T cell development. Nat. Rev. Immunol. 10, 657–663 (2010).
Haks, M.C. et al. Attenuation of γδ TCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity 22, 595–606 (2005).
Hayes, S.M., Li, L. & Love, P.E. TCR signal strength influences αβ/γδ lineage fate. Immunity 22, 583–593 (2005).
O'Brien, R.L. & Born, W.K. γδ T cell subsets: a link between TCR and function? Semin. Immunol. 22, 193–198 (2010).
Narayan, K. et al. & Immunological Genome Project Consortium. Intrathymic programming of effector fates in three molecularly distinct γδ T cell subtypes. Nat. Immunol. 13, 511–518 (2012).
Malhotra, N. et al. & Immunological Genome Project Consortium. A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity 38, 681–693 (2013).
Dave, V.P. et al. CD3δ deficiency arrests development of the αβ but not the γδ T cell lineage. EMBO J. 16, 1360–1370 (1997).
Coffey, F. et al. The TCR ligand-inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J. Exp. Med. 211, 329–343 (2014).
Azzam, H.S. et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188, 2301–2311 (1998).
Haas, J.D. et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37, 48–59 (2012).
Schmolka, N. et al. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory γδ T cell subsets. Nat. Immunol. 14, 1093–1100 (2013).
Schmolka, N., Wencker, M., Hayday, A.C. & Silva-Santos, B. Epigenetic and transcriptional regulation of γδ T cell differentiation: programming cells for responses in time and space. Semin. Immunol. 27, 19–25 (2015).
Silva-Santos, B., Pennington, D.J. & Hayday, A.C. Lymphotoxin-mediated regulation of γδ cell differentiation by αβ T cell progenitors. Science 307, 925–928 (2005).
Kannan, Y. et al. IκBζ augments IL-12- and IL-18-mediated IFN-γ production in human NK cells. Blood 117, 2855–2863 (2011).
Gu, X. et al. The gp49B1 inhibitory receptor regulates the IFN-gamma responses of T cells and NK cells. J. Immunol. 170, 4095–4101 (2003).
Ribot, J.C. et al. Cutting edge: adaptive versus innate receptor signals selectively control the pool sizes of murine IFN-γ– or IL-17-producing γδ T cells upon infection. J. Immunol. 185, 6421–6425 (2010).
D'Ombrain, M.C., Hansen, D.S., Simpson, K.M. & Schofield, L. γδ-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-γ response to Plasmodium falciparum malaria. Eur. J. Immunol. 37, 1864–1873 (2007).
Hayes, S.M. & Love, P.E. Stoichiometry of the murine γδ T cell receptor. J. Exp. Med. 203, 47–52 (2006).
Siegers, G.M. et al. Different composition of the human and the mouse γδ T cell receptor explains different phenotypes of CD3γ and CD3δ immunodeficiencies. J. Exp. Med. 204, 2537–2544 (2007).
Zapata, D.A. et al. Conformational and biochemical differences in the TCR-CD3 complex of CD8+ versus CD4+ mature lymphocytes revealed in the absence of CD3γ. J. Biol. Chem. 274, 35119–35128 (1999).
Fernández-Malavé, E. et al. Overlapping functions of human CD3δ and mouse CD3γ in αβ T-cell development revealed in a humanized CD3γ-mouse. Blood 108, 3420–3427 (2006).
Hayes, S.M. et al. Activation-induced modification in the CD3 complex of the γδ T cell receptor. J. Exp. Med. 196, 1355–1361 (2002).
Hayes, S.M. & Love, P.E. Distinct structure and signaling potential of the γδ TCR complex. Immunity 16, 827–838 (2002).
Roark, C.L. et al. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J. Immunol. 179, 5576–5583 (2007).
Seiler, M.P. et al. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat. Immunol. 13, 264–271 (2012).
Haks, M.C., Krimpenfort, P., Borst, J. & Kruisbeek, A.M. The CD3γ chain is essential for development of both the TCRαβ and TCRγδ lineages. EMBO J. 17, 1871–1882 (1998).
Liehl, P. et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat. Med. 20, 47–53 (2014).
R Development Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2011).
Huber, W. et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 (2015).
Carvalho, B.S. & Irizarry, R.A. A framework for oligonucleotide microarray preprocessing. Bioinformatics 26, 2363–2367 (2010).
Ritchie, M.E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Acknowledgements
We thank K. Serre, J. Martins, N. Schmolka, A. Amorim, V.Z. Luís and M.M. Mota (all at iMM Lisboa); and B. Garcillán, D. de Juan, M. Mazariegos, M. Sanz-Rodríguez and S. Diaz-Castroverde (Complutense University) for help and advice; A. Hayday (King's College London) and P. Pereira (Pasteur Institute) for insightful discussions; and the staff of the animal and flow cytometry facilities at iMM Lisboa and Complutense University for technical assistance. This work was funded by the European Research Council (StG_260352 and CoG_646701 to B.S.-S.); MINECO (SAF2011-24235 and BES-2012-055054 to J.R.R.), CAM (S2010/ BMD-2316/2326 to J.R.R.) and the Lair Foundation (2012/0070 to J.R.R.); and FIS PI11/02198 and MINECO SAF2014-54708-R to E.F.-M.
Author information
Authors and Affiliations
Contributions
M.M.-R., B.S.-S., E.F.-M., J.R.R. and D.J.P. designed research; M.M.-R., J.C.R., A.R.G., N.G.-S. and A.P. performed experiments; B.S.-S., E.F.-M. and J.R.R. supervised research and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
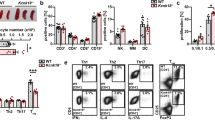
Supplementary Figure 1 γδ and αβ T cell development in CD3 mutant mice.
(a) Flow cytometry showing the CD3ɛ vs TCRδ phenotype of thymocytes from adult WT or CD3SH (single heterozygote for CD3δ or CD3γ) mice (n = 3 per group). Numbers within gated areas indicate % cells in each throughout. (b) Left, representative CD3ɛ vs TCRδ phenotype of thymocytes from the indicated adult mice. Numbers as in (a). Right, CD3ε and TCRδ expression (MFI) in gated TCRδ+ thymocytes and CD3+ thymocytes, respectively, from the indicated adult mice (n = 3 per group). Numbers indicate MFI (c) Gating strategy for CD3+ TCRδ+ γδ T cell identification. Doublets and dead cells were excluded. (d, e) Comparative analysis of TCRβ+CD1d+ NKT cells (d) and CD4+Foxp3+ Treg cells (e) in WT and CD3DH mice. Numbers in c, d, and e indicate percentage of cells within the marked region.
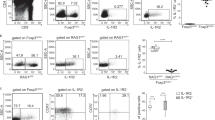
Supplementary Figure 2 γδ T cells from CD3DH mice show impaired TCRγδ signaling.
(a) Flow cytometry of phosphorylated AKT (empty histograms) in sorted TCRδ+CD3+CD27+ lymph node cells from the indicated mice, after 5 min stimulation with PMA plus ionomycin; filled histograms represent staining with isotype-matched control antibody. (b) Intracellular calcium mobilization in TCRδ+CD3+CD27+ lymph node cells obtained from the indicated mice, after 5 min stimulation with 10 µg/ml biotinylated anti-CD3ɛ mAb followed by TCR crosslinking with 10 µg/ml streptavidin, and then assessed over 5 min, followed by 2 min stimulation with PMA plus ionomycin.
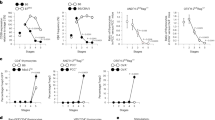
Supplementary Figure 3 Contributions of WT or CD3DH progenitors to γδ and CD4+ thymocytes in mixed bone marrow chimeras.
Thy-1.1+ (WT-derived) vs Thy-1.2+ (CD3DH-derived) fractions within TCRδ+CD3+ thymocytes (a) and CD4+CD3+ thymocytes (b) from 1:9 mixed WT:CD3DH BM chimeras. Each symbol indicates one individual host, either RAG2−/− (squares) or TCRδ−/− (triangles). *P < 0.01, **P < 0.001 (Student’s t-test). Data are from three independent experiments.
Supplementary Figure 4 Transcriptional signatures of Vγ-based γδ thymocyte subsets.
Relative expression of Egr3, Id2, Id3 and Gp49b (a) or Sox4, Sox13, Bcl11b and Blk (b), in arbitrary units normalized to the housekeeping gene hprt, in sorted Vγ1+, Vγ4+ or Vγ1−Vγ4− thymocytes from either WT or CD3DH E18 embryos. Data shown are the mean +/− s.d.
Supplementary Figure 5 CD4+ T cells from CD3DH mice differentiate normally into TH1 cells.
(a) Flow cytometry of intracellular IFN-γ expression of WT (top) and CD3DH (bottom) TCRβ+CD4+ splenocytes incubated for 4 days without stimuli (left panels) or on plate-bound α-CD3ɛ and α-CD28 mAb (5ng/ml each), without (middle panels) or with (right panels) Th1-polarizing cytokines IL-2 (10 ng/ml) and IL-12 (50 ng/ml) plus anti-IL-4 (10 µg/mL) neutralizing antibody. Numbers indicate % of cells in each quadrant. Data are representative of two independent experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 and Supplementary Table 1 (PDF 1624 kb)
Rights and permissions
About this article
Cite this article
Muñoz-Ruiz, M., Ribot, J., Grosso, A. et al. TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets. Nat Immunol 17, 721–727 (2016). https://doi.org/10.1038/ni.3424
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3424