Abstract
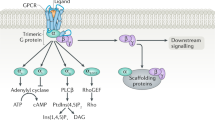
Most clinically validated drugs for treating patients with cardiovascular disease target G-protein-coupled receptors (GPCRs) in the cell membrane. GPCRs engage and activate multiple intracellular signaling cascades, which are regulated by serine/threonine protein kinases. These protein kinases are cytoplasmic, more abundant than GPCRs, and have rapidly emerged as drug targets in cardiovascular diseases. One exciting potential advantage to targeting serine/threonine protein kinases rather than GPCRs is the capability of influencing more precisely the diverse biological responses that are initiated by a common GPCR. On the other hand, highly specific targeting of individual protein kinases for drug therapy presents some medicinal chemistry challenges. This concise review focuses on the biology of serine/threonine protein kinases in the cardiovascular system, discusses the current state of protein kinase inhibitor drug development for myocardial diseases, and illustrates some of the unique medicinal chemistry considerations in targeting protein kinases.
Key Points
-
Despite abundance, interest in targeting protein kinases for myocardial disease developed later than that for G-protein-coupled receptors
-
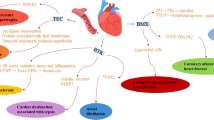
In myocardial diseases overexpression of protein kinases can recapitulate clinically important disease phenotypes, while inhibition of protein kinases can reduce susceptibility
-
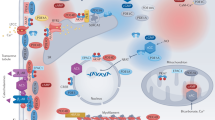
Potential strategies for protein kinase inhibition include competition for ATP binding, prevention of 'anchoring' to target proteins, and sterically induced reduction in ATP binding affinity and stabilization of inactive conformations
-
A potential advantage for targeting protein kinases over G-protein-coupled receptors is improved biological specificity, but this feature remains to be validated clinically
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cohen P (2002) Protein kinases—the major drug targets of the twenty-first century? Nat Rev Drug Discov 1: 309–315
Force T et al. (2004) Inhibitors of protein kinase signaling pathways: emerging therapies for cardiovascular disease. Circulation 109: 1196–1205
Molkentin JD et al. (1998) A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228
Zhang R et al. (2005) Calmodulin kinase II inhibition protects against structural heart disease. Nat Med 11: 409–417
Bers DM (2005) Beyond beta blockers. Nat Med 11: 379–380
Puceat M et al. (1994) Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem 269: 16938–16944
Muller JG et al. (2002) Differential regulation of the cardiac sodium calcium exchanger promoter in adult and neonatal cardiomyocytes by Nkx2.5 and serum response factor. J Mol Cell Cardiol 34: 807–821
Johnson SA and Hunter T (2005) Kinomics: methods for deciphering the kinome. Nat Methods 2: 17–25
Gollob MH et al. (2001) Identification of a gene responsible for familial Wolff–Parkinson–White syndrome. N Engl J Med 344: 1823–1831
De Koninck P and Schulman H (1998) Sensitivity of CaM kinase II to the frequency of Ca2 oscillations. Science 279: 227–230
Lipinski CA et al. (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 46: 3–26
Manning G et al. (2002) The protein kinase complement of the human genome. Science 298: 1912–1934
Martin EJ and Critchlow RE (1999) Beyond mere diversity: tailoring combinatorial libraries for drug discovery. J Comb Chem 1: 32–45
Schuffenhauer A et al. (2005) Library design for fragment based screening. Curr Top Med Chem 5: 751–762
Noble ME et al. (2004) Protein kinase inhibitors: insights into drug design from structure. Science 303: 1800–1805
Rosenberg OS et al. (2005) Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell 123: 849–860
Fabian MA et al. (2005) A small molecule–kinase interaction map for clinical kinase inhibitors. Nat Biotechnol 23: 329–336
Huse M and Kuriyan J (2002) The conformational plasticity of protein kinases. Cell 109: 275–282
Alessi DR et al. (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270: 27489–27494
Hudmon A et al. (2005) CaMKII tethers to L-type Ca2 channels, establishing a local and dedicated integrator of Ca2 signals for facilitation. J Cell Biol 171: 537–547
Tsui J et al. (2005) Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. J Biol Chem 280: 9210–9216
Vlahos CJ et al. (2003) Kinases as therapeutic targets for heart failure. Nat Rev Drug Discov 2: 99–113
Marx SO et al. (2000) PKA phosphorylation dissociates FKBP126 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376
Antos CL et al. (2001) Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase A. Circ Res 89: 997–1004
Hoch B et al. (1999) Identification and expression of δ-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res 84: 713–721
Zhang T et al. (2003) The δC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res 92: 912–919
Bowling N et al. (1999) Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation 99: 384–391
Tian R et al. (1999) Long-term expression of protein kinase C in adult mouse hearts improves postischemic recovery. Proc Natl Acad Sci USA 96: 13536–13541
Dorn GW et al. (1999) Sustained in vivo cardiac protection by a rationally designed peptide that causes ε protein kinase C translocation. Proc Natl Acad Sci USA 96: 12798–12803
Cook SA et al. (1999) Activation of c-Jun N-terminal kinases and p38-mitogen-activated protein kinases in human heart failure secondary to ischaemic heart disease. J Mol Cell Cardiol 31: 1429–1434
Bueno OF et al. (2000) The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J 19: 6341–6350
Flesch M et al. (2001) Differential regulation of mitogen-activated protein kinases in the failing human heart in response to mechanical unloading. Circulation 104: 2273–2276
Liao P et al. (2001) The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA 98: 12283–12288
Zhang S et al. (2003) The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest 111: 833–841
Braz JC et al. (2003) Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest 111: 1475–1486
Petrich BG et al. (2003) Temporal activation of c-Jun N-terminal kinase in adult transgenic heart via cre-loxP-mediated DNA recombination. FASEB J 17: 749–751
Liang Q et al. (2003) c-Jun N-terminal kinases (JNK) antagonize cardiac growth through cross-talk with calcineurin-NFAT signaling. EMBO J 22: 5079–5089
Podewski EK et al. (2003) Alterations in Janus kinase (JAK)-signal transducers and activators of transcription (STAT) signaling in patients with end-stage dilated cardiomyopathy. Circulation 107: 798–802
Harada M et al. (2005) G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes Nat Med 11: 305–311
Xuan YT et al. (2001) An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA 98: 9050–9055
Baba HA et al. (2003) Dynamic regulation of MEK/Erks and Akt/GSK-3β in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res 59: 390–399
Fujio Y et al. (2000) Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667
Nagoshi T et al. (2005) PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 115: 2128–2138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Mark E Anderson and Howard Shulman are named inventors on a patent to treat cardiac arrhythmias by CaMKII inhibition.
Linda S Higgins directs drug development and efforts to inhibit kinases, including studies into CaMKII, at Scios Inc., Fremont, CA, USA.
Rights and permissions
About this article
Cite this article
Anderson, M., Higgins, L. & Schulman, H. Disease mechanisms and emerging therapies: protein kinases and their inhibitors in myocardial disease. Nat Rev Cardiol 3, 437–445 (2006). https://doi.org/10.1038/ncpcardio0585
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0585
This article is cited by
-
Targeting ryanodine receptors for anti-arrhythmic therapy
Acta Pharmacologica Sinica (2011)
-
Rescuing a failing heart: think globally, treat locally
Nature Medicine (2009)
-
Mechanisms of Disease: ion channel remodeling in the failing ventricle
Nature Clinical Practice Cardiovascular Medicine (2008)
-
Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface
Nature Reviews Drug Discovery (2007)